Abstract
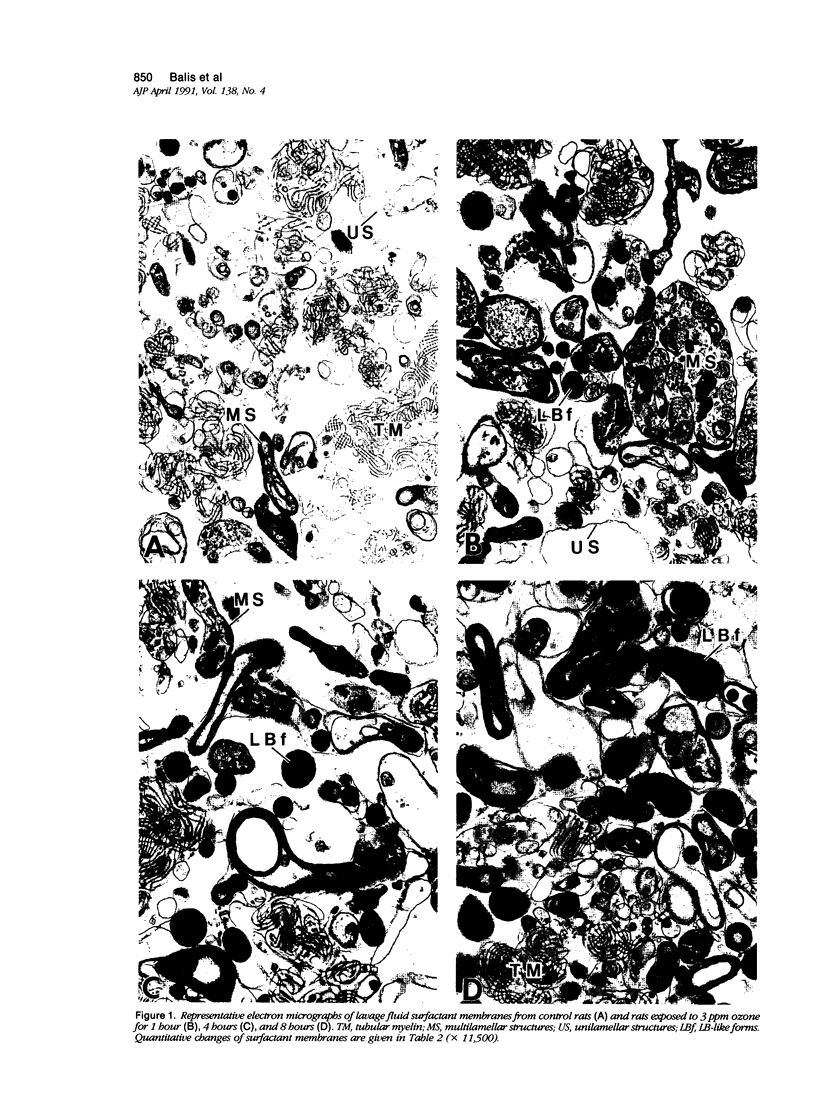
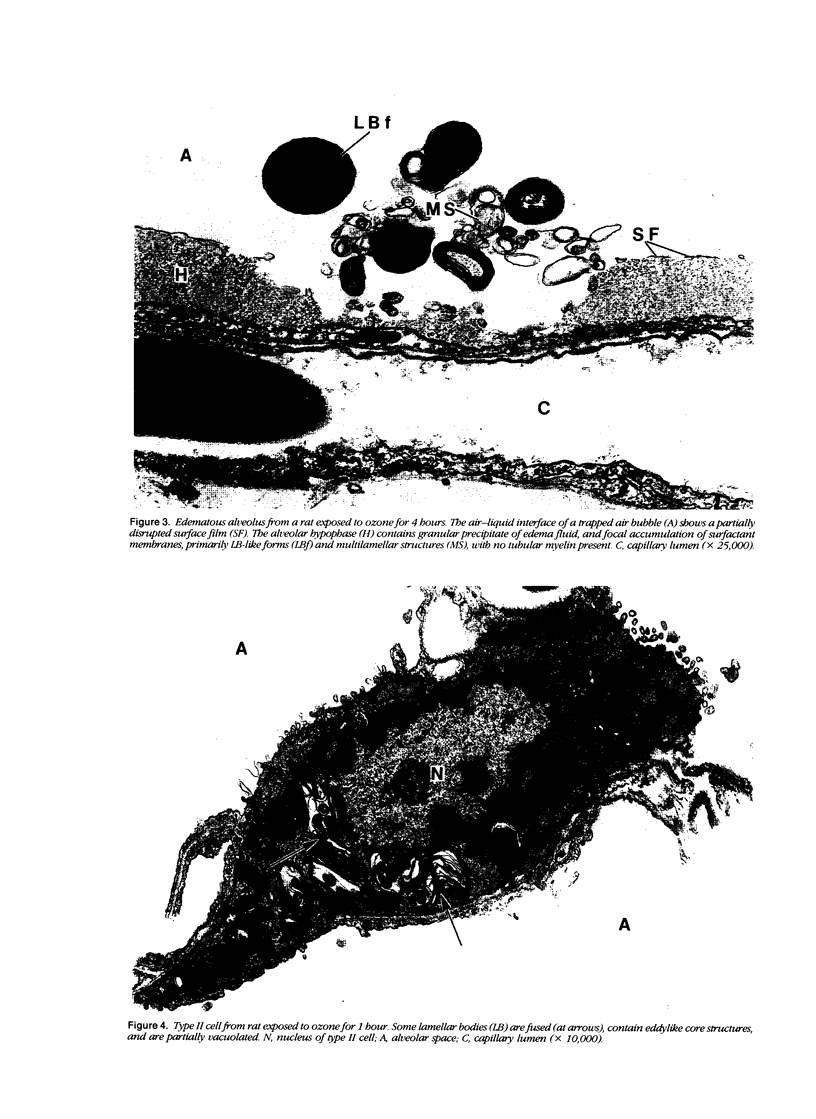
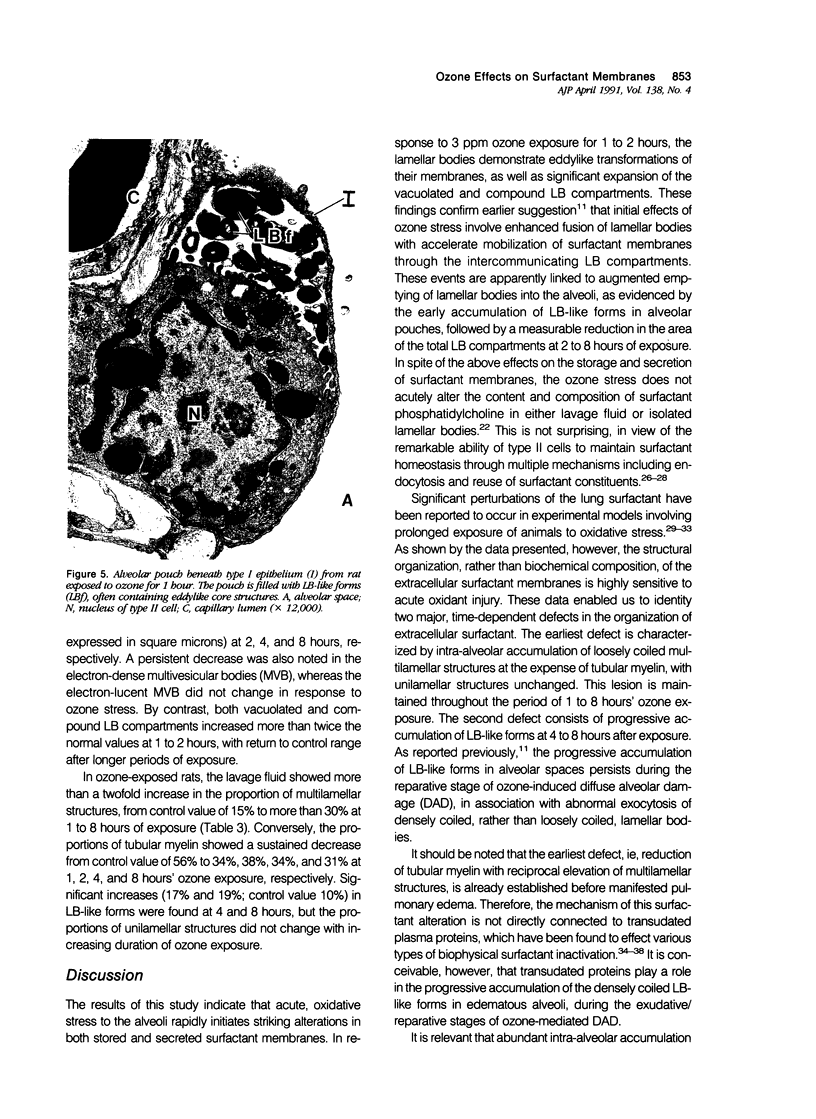
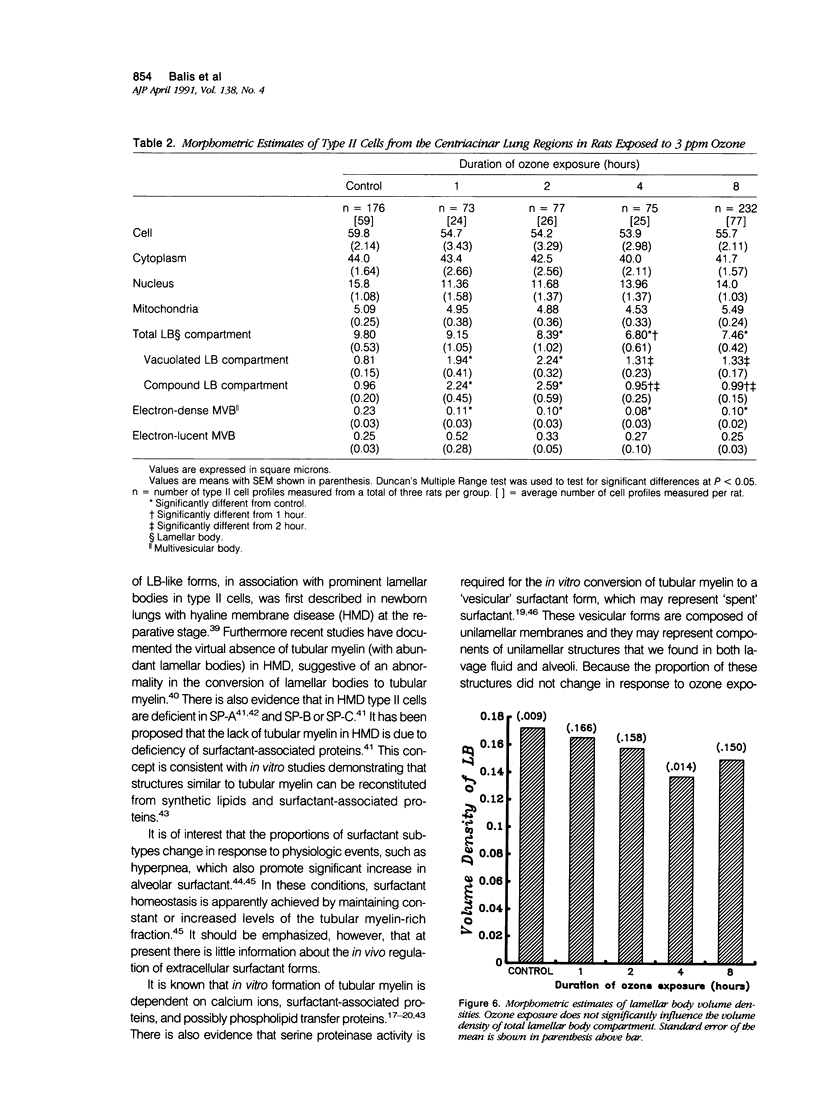
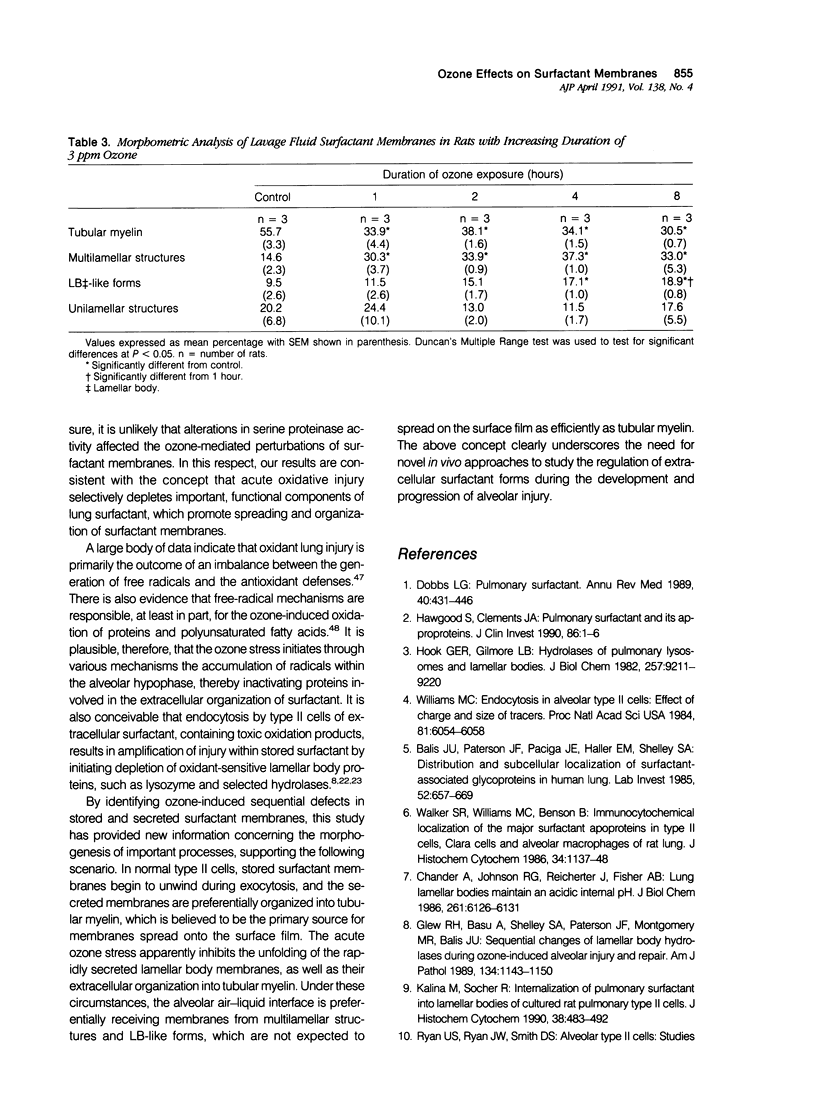
To identify the early changes of surfactant secretion in response to acute oxidant stress, the authors evaluated morphometrically centriacinar type II cells and lavage fluid surfactant forms obtained immediately after exposure of adult rats to 3 ppm ozone for 1, 2, 4, or 8 hours. In this model, the rat lung develops progressive alveolar edema with significant elevation of lavage fluid proteins at 2 to 8 hours of exposure. Ultrastructural changes in type II cells at 1 and 2 hours included enhanced lamellar body (LB) fusion with significant increase in the compound and vacuolated LB compartments. Parallel changes of lavage fluid surfactant membranes included a sustained, twofold increase in the proportion of loosely coiled multilamellar structures at 1 to 8 hours, with reciprocal decrease in the proportion of tubular myelin from control value of 56% to 34%. The proportion of densely coiled LB-like forms in lavage fluid increased significantly at 4 and 8 hours, whereas the proportions of unilamellar structures remained unchanged. The results indicate that ozone-induced alveolar injury initiates time-dependent defects in the organization of stored and secreted surfactant membranes. The acute ozone stress inhibits unfolding of secreted lamellar body membranes as well as their organization into tubular myelin, thereby perturbing the proportions of extracellular surfactant membranes that are available for adsorption onto the surface film.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balis J. U., Delivoria M., Conen P. E. Maturation of postnatal human lung and the idiopathic respiratory distress syndrome. Lab Invest. 1966 Mar;15(3):530–546. [PubMed] [Google Scholar]
- Balis J. U., Paterson J. F., Haller E. M., Shelley S. A., Montgomery M. R. Ozone-induced lamellar body responses in a rat model for alveolar injury and repair. Am J Pathol. 1988 Aug;132(2):330–344. [PMC free article] [PubMed] [Google Scholar]
- Balis J. U., Paterson J. F., Paciga J. E., Haller E. M., Shelley S. A. Distribution and subcellular localization of surfactant-associated glycoproteins in human lung. Lab Invest. 1985 Jun;52(6):657–669. [PubMed] [Google Scholar]
- Balis J. U., Shelley S. A., McCue M. J., Rappaport E. S. Mechanisms of damage to the lung surfactant system. Ultrastructure and quantitation of normal and in vitro inactivated lung surfactant. Exp Mol Pathol. 1971 Apr;14(2):243–262. doi: 10.1016/0014-4800(71)90069-4. [DOI] [PubMed] [Google Scholar]
- Chander A., Johnson R. G., Reicherter J., Fisher A. B. Lung lamellar bodies maintain an acidic internal pH. J Biol Chem. 1986 May 5;261(13):6126–6131. [PubMed] [Google Scholar]
- Dobbs L. G. Pulmonary surfactant. Annu Rev Med. 1989;40:431–446. doi: 10.1146/annurev.me.40.020189.002243. [DOI] [PubMed] [Google Scholar]
- Eckenhoff R. G. Perinatal changes in lung surfactant calcium measured in situ. J Clin Invest. 1989 Oct;84(4):1295–1301. doi: 10.1172/JCI114297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glew R. H., Basu A., Shelley S. A., Paterson J. F., Diven W. F., Montgomery M. R., Balis J. U. Sequential changes of lamellar body hydrolases during ozone-induced alveolar injury and repair. Am J Pathol. 1989 May;134(5):1143–1150. [PMC free article] [PubMed] [Google Scholar]
- Gross N. J., Narine K. R. Surfactant subtypes in mice: characterization and quantitation. J Appl Physiol (1985) 1989 Jan;66(1):342–349. doi: 10.1152/jappl.1989.66.1.342. [DOI] [PubMed] [Google Scholar]
- Gross N. J., Schultz R. M. Serine proteinase requirement for the extra-cellular metabolism of pulmonary surfactant. Biochim Biophys Acta. 1990 May 22;1044(2):222–230. doi: 10.1016/0005-2760(90)90306-i. [DOI] [PubMed] [Google Scholar]
- Gross N. J., Smith D. M. Impaired surfactant phospholipid metabolism in hyperoxic mouse lungs. J Appl Physiol Respir Environ Exerc Physiol. 1981 Nov;51(5):1198–1203. doi: 10.1152/jappl.1981.51.5.1198. [DOI] [PubMed] [Google Scholar]
- Haagsman H. P., Hawgood S., Sargeant T., Buckley D., White R. T., Drickamer K., Benson B. J. The major lung surfactant protein, SP 28-36, is a calcium-dependent, carbohydrate-binding protein. J Biol Chem. 1987 Oct 15;262(29):13877–13880. [PubMed] [Google Scholar]
- Hallman M., Epstein B. L., Gluck L. Analysis of labeling and clearance of lung surfactant phospholipids in rabbit. Evidence of bidirectional surfactant flux between lamellar bodies and alveolar lavage. J Clin Invest. 1981 Sep;68(3):742–751. doi: 10.1172/JCI110310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawgood S., Clements J. A. Pulmonary surfactant and its apoproteins. J Clin Invest. 1990 Jul;86(1):1–6. doi: 10.1172/JCI114670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm B. A., Notter R. H., Finkelstein J. N. Surface property changes from interactions of albumin with natural lung surfactant and extracted lung lipids. Chem Phys Lipids. 1985 Sep;38(3):287–298. doi: 10.1016/0009-3084(85)90022-2. [DOI] [PubMed] [Google Scholar]
- Holm B. A., Notter R. H., Siegle J., Matalon S. Pulmonary physiological and surfactant changes during injury and recovery from hyperoxia. J Appl Physiol (1985) 1985 Nov;59(5):1402–1409. doi: 10.1152/jappl.1985.59.5.1402. [DOI] [PubMed] [Google Scholar]
- Hook G. E., Gilmore L. B. Hydrolases of pulmonary lysosomes and lamellar bodies. J Biol Chem. 1982 Aug 10;257(15):9211–9220. [PubMed] [Google Scholar]
- Jacobs H. C., Ikegami M., Jobe A. H., Berry D. D., Jones S. Reutilization of surfactant phosphatidylcholine in adult rabbits. Biochim Biophys Acta. 1985 Oct 23;837(1):77–84. doi: 10.1016/0005-2760(85)90087-6. [DOI] [PubMed] [Google Scholar]
- Kalina M., Socher R. Internalization of pulmonary surfactant into lamellar bodies of cultured rat pulmonary type II cells. J Histochem Cytochem. 1990 Apr;38(4):483–492. doi: 10.1177/38.4.2156921. [DOI] [PubMed] [Google Scholar]
- Keough K. M., Parsons C. S., Tweeddale M. G. Interactions between plasma proteins and pulmonary surfactant: pulsating bubble studies. Can J Physiol Pharmacol. 1989 Jun;67(6):663–668. doi: 10.1139/y89-106. [DOI] [PubMed] [Google Scholar]
- King R. J., Coalson J. J., Seidenfeld J. J., Anzueto A. R., Smith D. B., Peters J. I. O2- and pneumonia-induced lung injury. II. Properties of pulmonary surfactant. J Appl Physiol (1985) 1989 Jul;67(1):357–365. doi: 10.1152/jappl.1989.67.1.357. [DOI] [PubMed] [Google Scholar]
- Lumb R. H., Benson B. J., Clements J. A. Transfer of phospholipids by a protein fraction obtained from canine pulmonary lavage. Biochim Biophys Acta. 1988 Dec 16;963(3):549–552. doi: 10.1016/0005-2760(88)90324-4. [DOI] [PubMed] [Google Scholar]
- Magoon M. W., Wright J. R., Baritussio A., Williams M. C., Goerke J., Benson B. J., Hamilton R. L., Clements J. A. Subfractionation of lung surfactant. Implications for metabolism and surface activity. Biochim Biophys Acta. 1983 Jan 7;750(1):18–31. doi: 10.1016/0005-2760(83)90200-x. [DOI] [PubMed] [Google Scholar]
- Manabe T. Freeze-fracture study of alveolar lining layer in adult rat lungs. J Ultrastruct Res. 1979 Oct;69(1):86–97. doi: 10.1016/s0022-5320(79)80044-1. [DOI] [PubMed] [Google Scholar]
- Margraf L. R., Paciga J. E., Balis J. U. Surfactant-associated glycoproteins accumulate in alveolar cells and secretions during reparative stage of hyaline membrane disease. Hum Pathol. 1990 Apr;21(4):392–396. doi: 10.1016/0046-8177(90)90200-o. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Tolbert N. E., Bieber L. L. Protein determination in membrane and lipoprotein samples: manual and automated procedures. Methods Enzymol. 1981;72:296–303. doi: 10.1016/s0076-6879(81)72018-4. [DOI] [PubMed] [Google Scholar]
- Matalon S., Holm B. A., Notter R. H. Mitigation of pulmonary hyperoxic injury by administration of exogenous surfactant. J Appl Physiol (1985) 1987 Feb;62(2):756–761. doi: 10.1152/jappl.1987.62.2.756. [DOI] [PubMed] [Google Scholar]
- Menzel D. B. Ozone: an overview of its toxicity in man and animals. J Toxicol Environ Health. 1984;13(2-3):183–204. [PubMed] [Google Scholar]
- Montgomery M. R., Anderson R. E., Mortenson G. A. A compact, versatile inhalation exposure chamber for small animal studies. Lab Anim Sci. 1976 Jun;26(3):461–464. [PubMed] [Google Scholar]
- Nicholas T. E., Power J. H., Barr H. A. The pulmonary consequences of a deep breath. Respir Physiol. 1982 Sep;49(3):315–324. doi: 10.1016/0034-5687(82)90119-0. [DOI] [PubMed] [Google Scholar]
- Notter R. H., Penney D. P., Finkelstein J. N., Shapiro D. L. Adsorption of natural lung surfactant and phospholipid extracts related to tubular myelin formation. Pediatr Res. 1986 Jan;20(1):97–101. doi: 10.1203/00006450-198601000-00026. [DOI] [PubMed] [Google Scholar]
- Power J. H., Barr H. A., Jones M. E., Nicholas T. E. Changes in surfactant pools after a physiological increase in alveolar surfactant. J Appl Physiol (1985) 1987 Nov;63(5):1902–1911. doi: 10.1152/jappl.1987.63.5.1902. [DOI] [PubMed] [Google Scholar]
- Sanders R. L., Hassett R. J., Vatter A. E. Isolation of lung lamellar bodies and their conversion to tubular myelin figures in vitro. Anat Rec. 1980 Nov;198(3):485–501. doi: 10.1002/ar.1091980310. [DOI] [PubMed] [Google Scholar]
- Sanderson R. J., Vatter A. E. A mode of formation of tubular myelin from lamellar bodies in the lung. J Cell Biol. 1977 Sep;74(3):1027–1031. doi: 10.1083/jcb.74.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger W., Stöhr G., Wolf H. R., Neuhof H. Alteration of surfactant function due to protein leakage: special interaction with fibrin monomer. J Appl Physiol (1985) 1985 Feb;58(2):326–338. doi: 10.1152/jappl.1985.58.2.326. [DOI] [PubMed] [Google Scholar]
- Shelley S. A., Paciga J. E., Paterson J. F., Balis J. U. Ozone-induced alterations of lamellar body lipid and protein during alveolar injury and repair. Lipids. 1989 Sep;24(9):769–774. doi: 10.1007/BF02544582. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Fujita Y., Kogishi K. Reconstitution of tubular myelin from synthetic lipids and proteins associated with pig pulmonary surfactant. Am Rev Respir Dis. 1989 Jul;140(1):75–81. doi: 10.1164/ajrccm/140.1.75. [DOI] [PubMed] [Google Scholar]
- Walker S. R., Williams M. C., Benson B. Immunocytochemical localization of the major surfactant apoproteins in type II cells, Clara cells, and alveolar macrophages of rat lung. J Histochem Cytochem. 1986 Sep;34(9):1137–1148. doi: 10.1177/34.9.2426341. [DOI] [PubMed] [Google Scholar]
- Williams M. C. Conversion of lamellar body membranes into tubular myelin in alveoli of fetal rat lungs. J Cell Biol. 1977 Feb;72(2):260–277. doi: 10.1083/jcb.72.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M. C. Endocytosis in alveolar type II cells: effect of charge and size of tracers. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6054–6058. doi: 10.1073/pnas.81.19.6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. L., Crapo J. D., Kremers S. A., Brumley G. W. Pulmonary surfactant lipid production in oxygen-exposed rat lungs. Lab Invest. 1982 Jun;46(6):570–576. [PubMed] [Google Scholar]
- Young S. L., Wright J. R., Clements J. A. Cellular uptake and processing of surfactant lipids and apoprotein SP-A by rat lung. J Appl Physiol (1985) 1989 Mar;66(3):1336–1342. doi: 10.1152/jappl.1989.66.3.1336. [DOI] [PubMed] [Google Scholar]
- deMello D. E., Chi E. Y., Doo E., Lagunoff D. Absence of tubular myelin in lungs of infants dying with hyaline membrane disease. Am J Pathol. 1987 Apr;127(1):131–139. [PMC free article] [PubMed] [Google Scholar]
- deMello D. E., Phelps D. S., Patel G., Floros J., Lagunoff D. Expression of the 35kDa and low molecular weight surfactant-associated proteins in the lungs of infants dying with respiratory distress syndrome. Am J Pathol. 1989 Jun;134(6):1285–1293. [PMC free article] [PubMed] [Google Scholar]