Abstract
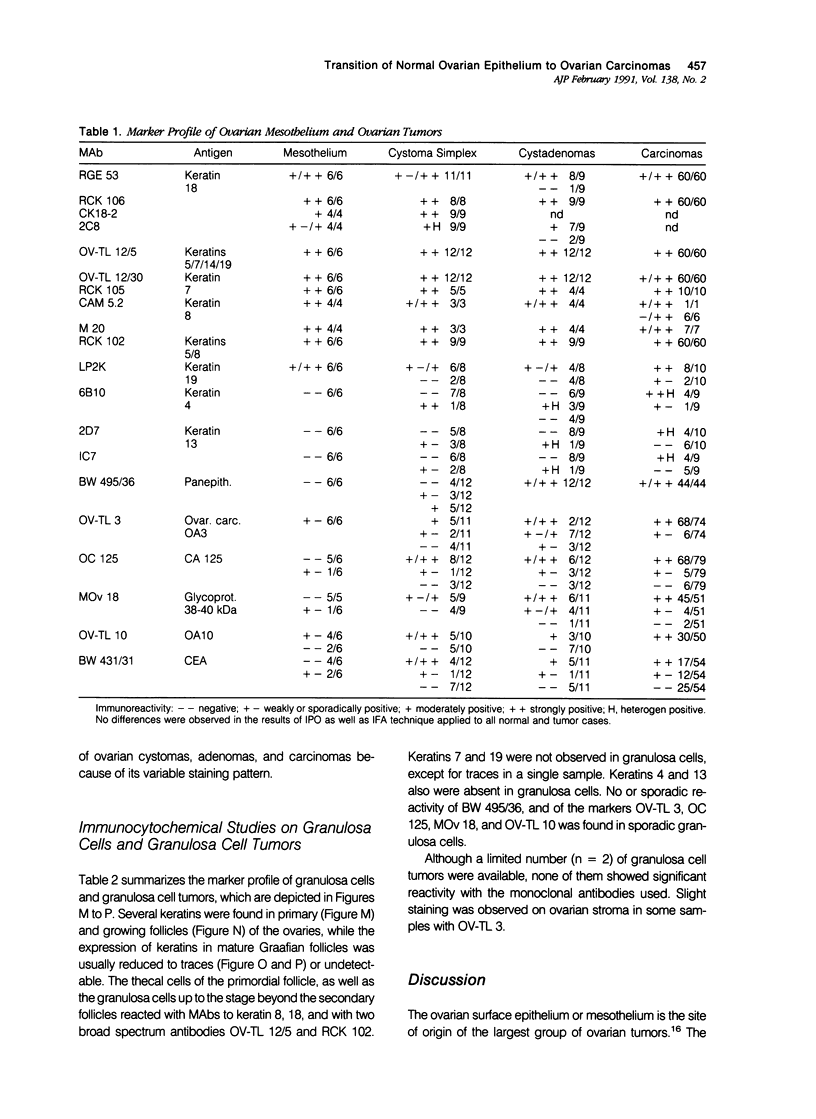
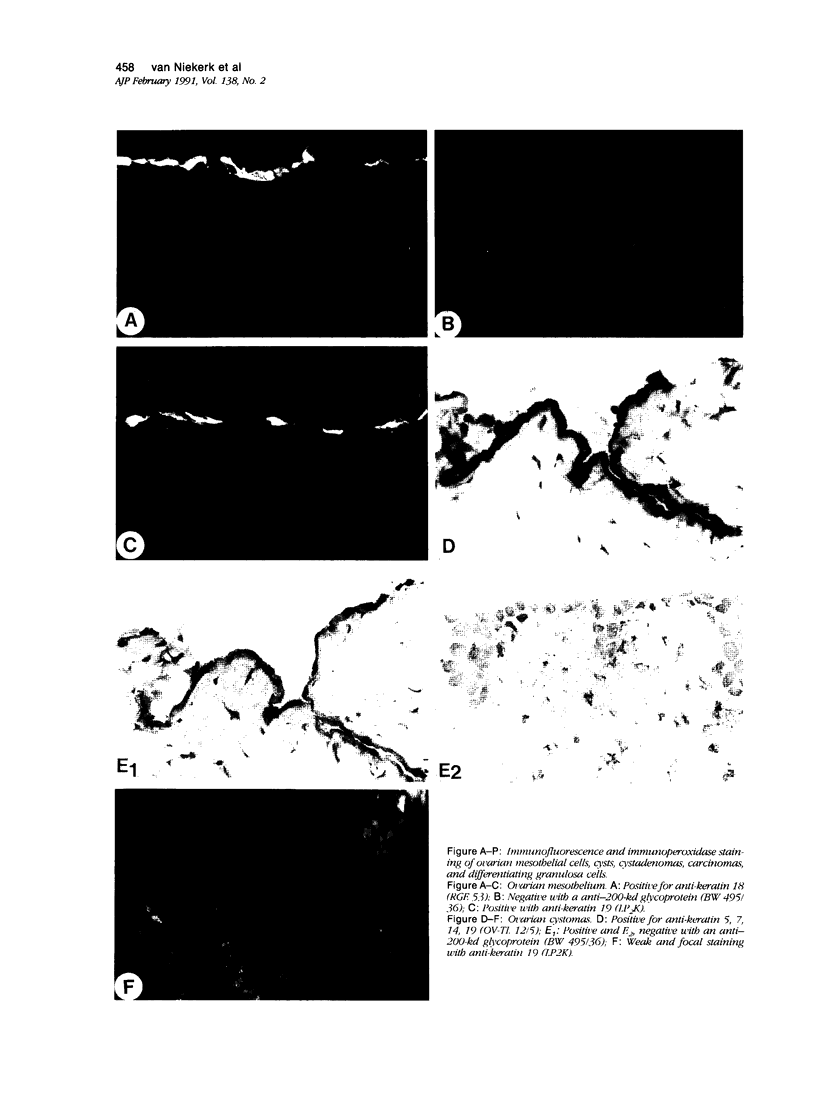
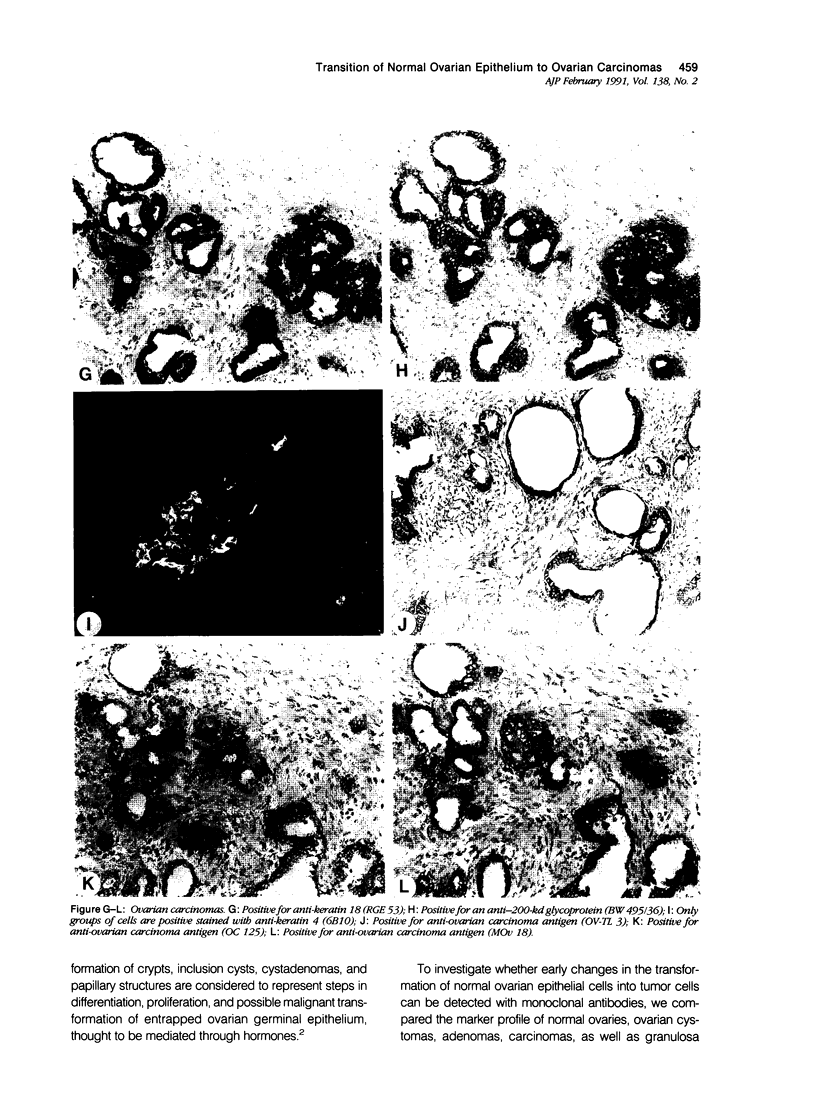
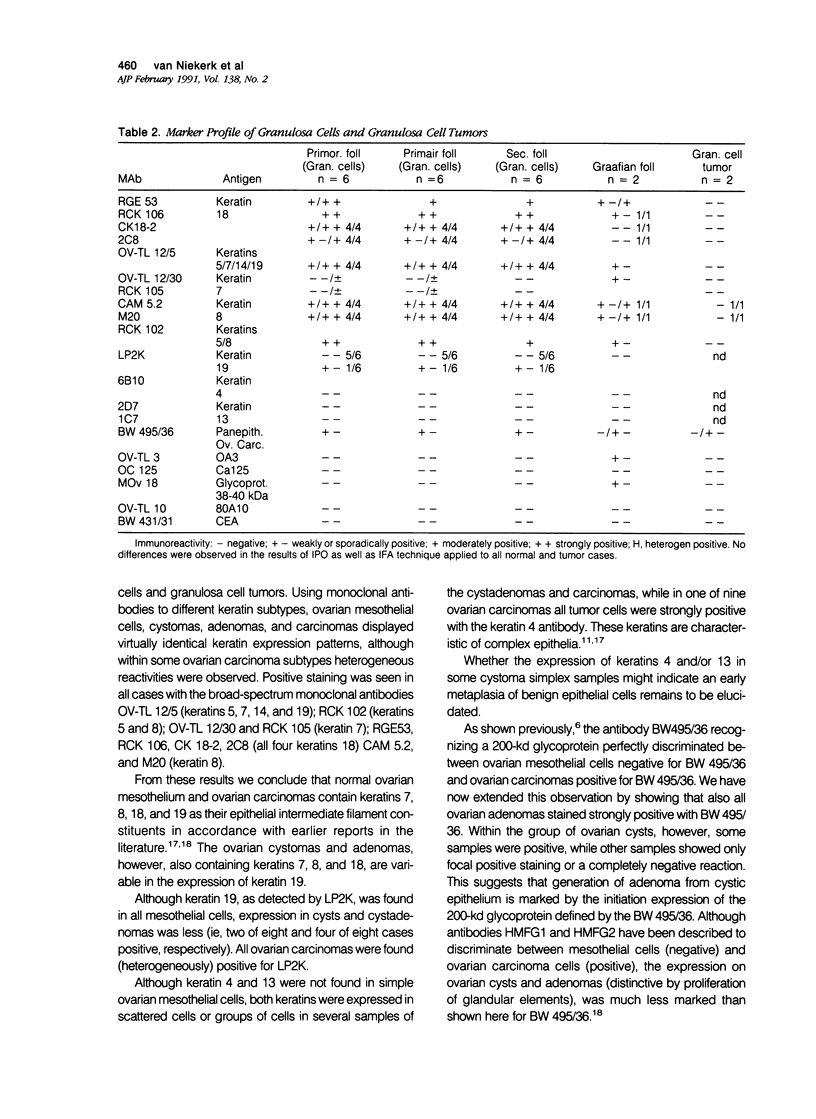
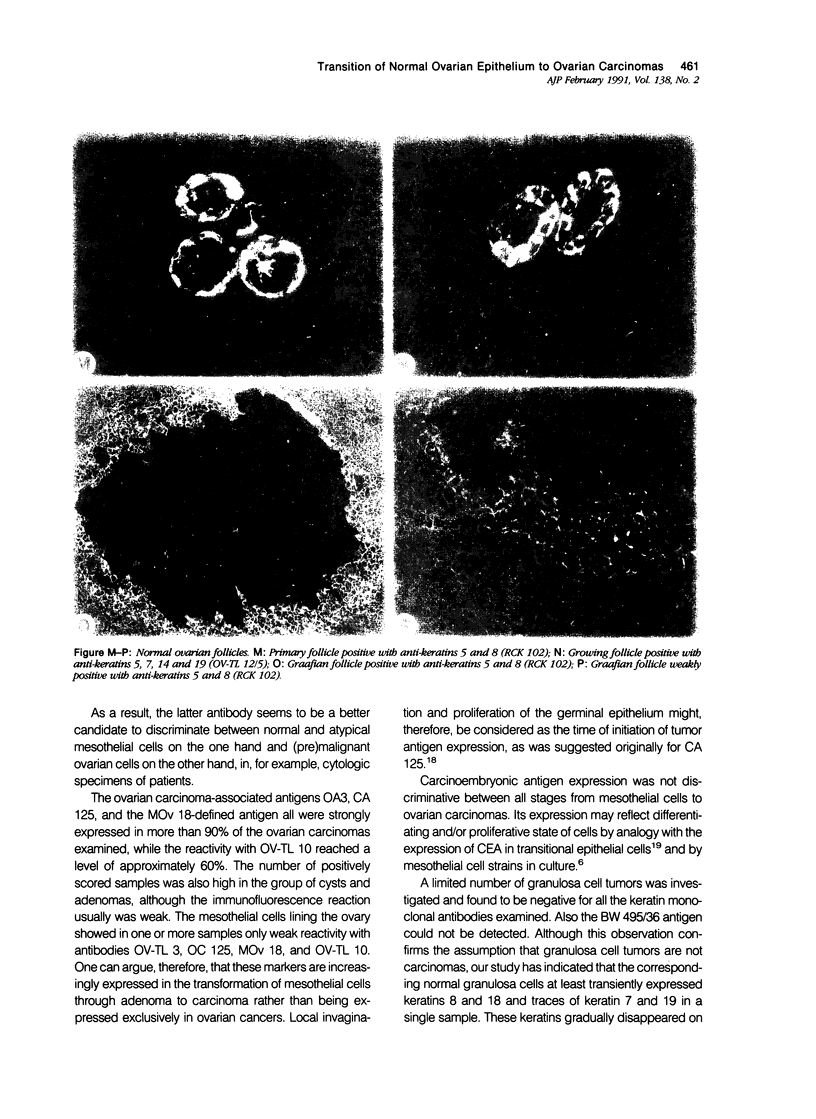
To investigate whether early changes in the transformation of normal ovarian epithelial cells into tumor cells can be detected with monoclonal antibodies, a comparative immunohistochemical study was performed on normal human ovarian mesothelial cells, cystomas, cystadenomas, ovarian carcinomas, as well as granulosa cell tumor. Using monoclonal antibodies against different keratin subtypes, it was shown that mesothelial cells, ovarian cysts, cystadenomas, and carcinomas all reacted positively with broad-spectrum anti-keratin monoclonal antibodies (MAbs), as well as with MAbs to keratins 7, 8, 18, and 19. Keratins 4 and 13 were not found in mesothelial cells, but positive groups of cells were identified in several cystomas, adenomas, and carcinomas. While mesothelial cells did not react with the pan-epithelial marker BW495/36, invaginating metaplastic mesothelial cells, inclusion cysts, cystomas, adenomas, and carcinomas showed an increasing reactivity with BW495/36, with an increasing degree of malignancy. The reactivity of MAbs against ovarian carcinoma-associated antigens (OV-TL 3, OC 125, MOv 18, and OV-TL 10) was limited to weak staining reaction in some mesothelial cells but were found to be positive on more than 50% of the ovarian cystadenomas and more than 90% of the ovarian carcinomas. Thecal and granulosa cells of primordial, primary, and secondary follicles all reacted positively with antibodies to the broad-spectrum keratins OV-TL 12/5 and RCK 102, and to keratins 8 and 18, but not with keratins 4, 7, 13, and 19. These keratins decreased or disappeared in granulosa cells of mature follicles (Graafian follicles), whereas granulosa cell tumors did not react with anti-keratin antibodies. The reactivity of BW 495/36 was negative or limited to traces in some granulosa cells. Ovarian carcinoma-associated antigens were not expressed in granulosa cells or granulosa cell tumors. The data indicate that mesothelial cells undergoing metaplastic changes finally resulting in ovarian cystadenomas (and carcinomas) initiate the synthesis of a 200-kd glycoprotein recognized by MAb (BW 495/36), the production of ovarian carcinoma associated antigens, in addition to focal production of keratin 4 and/or 13, as seen in several samples. The granulosa cell tumors decrease or switch off their keratin production and remain negative for the 200-kd glycoprotein and the ovarian carcinoma-associated antigens.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bast R. C., Jr, Feeney M., Lazarus H., Nadler L. M., Colvin R. B., Knapp R. C. Reactivity of a monoclonal antibody with human ovarian carcinoma. J Clin Invest. 1981 Nov;68(5):1331–1337. doi: 10.1172/JCI110380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Amsterdam A. Regulation of cytoskeletal protein organization and expression in human granulosa cells in response to gonadotropin treatment. Endocrinology. 1989 Feb;124(2):1033–1041. doi: 10.1210/endo-124-2-1033. [DOI] [PubMed] [Google Scholar]
- Blaustein A. Surface (germinal) epithelium and related ovarian neoplasms. Pathol Annu. 1981;16(Pt 1):247–294. [PubMed] [Google Scholar]
- Cramer D. W., Hutchison G. B., Welch W. R., Scully R. E., Ryan K. J. Determinants of ovarian cancer risk. I. Reproductive experiences and family history. J Natl Cancer Inst. 1983 Oct;71(4):711–716. [PubMed] [Google Scholar]
- Czernobilsky B., Moll R., Levy R., Franke W. W. Co-expression of cytokeratin and vimentin filaments in mesothelial, granulosa and rete ovarii cells of the human ovary. Eur J Cell Biol. 1985 May;37:175–190. [PubMed] [Google Scholar]
- Miotti S., Canevari S., Ménard S., Mezzanzanica D., Porro G., Pupa S. M., Regazzoni M., Tagliabue E., Colnaghi M. I. Characterization of human ovarian carcinoma-associated antigens defined by novel monoclonal antibodies with tumor-restricted specificity. Int J Cancer. 1987 Mar 15;39(3):297–303. doi: 10.1002/ijc.2910390306. [DOI] [PubMed] [Google Scholar]
- Moll R., Levy R., Czernobilsky B., Hohlweg-Majert P., Dallenbach-Hellweg G., Franke W. W. Cytokeratins of normal epithelia and some neoplasms of the female genital tract. Lab Invest. 1983 Nov;49(5):599–610. [PubMed] [Google Scholar]
- Nouwen E. J., Hendrix P. G., Dauwe S., Eerdekens M. W., De Broe M. E. Tumor markers in the human ovary and its neoplasms. A comparative immunohistochemical study. Am J Pathol. 1987 Feb;126(2):230–242. [PMC free article] [PubMed] [Google Scholar]
- Poels L. G., Peters D., van Megen Y., Vooijs G. P., Verheyen R. N., Willemen A., van Niekerk C. C., Jap P. H., Mungyer G., Kenemans P. Monoclonal antibody against human ovarian tumor-associated antigens. J Natl Cancer Inst. 1986 May;76(5):781–791. [PubMed] [Google Scholar]
- Ramaekers F., Huysmans A., Moesker O., Kant A., Jap P., Herman C., Vooijs P. Monoclonal antibody to keratin filaments, specific for glandular epithelia and their tumors. Use in surgical pathology. Lab Invest. 1983 Sep;49(3):353–361. [PubMed] [Google Scholar]
- Ramaekers F., Huysmans A., Schaart G., Moesker O., Vooijs P. Tissue distribution of keratin 7 as monitored by a monoclonal antibody. Exp Cell Res. 1987 May;170(1):235–249. doi: 10.1016/0014-4827(87)90133-9. [DOI] [PubMed] [Google Scholar]
- Ramaekers F., van Niekerk C., Poels L., Schaafsma E., Huijsmans A., Robben H., Schaart G., Vooijs P. Use of monoclonal antibodies to keratin 7 in the differential diagnosis of adenocarcinomas. Am J Pathol. 1990 Mar;136(3):641–655. [PMC free article] [PubMed] [Google Scholar]
- Shevchuk M. M., Fenoglio C. M., Richart R. M. Carcinoembryonic antigen localization in benign and malignant transitional epithelium. Cancer. 1981 Mar 1;47(5):899–905. doi: 10.1002/1097-0142(19810301)47:5<899::aid-cncr2820470515>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Smedts F., Ramaekers F., Robben H., Pruszczynski M., van Muijen G., Lane B., Leigh I., Vooijs P. Changing patterns of keratin expression during progression of cervical intraepithelial neoplasia. Am J Pathol. 1990 Mar;136(3):657–668. [PMC free article] [PubMed] [Google Scholar]
- Stasiak P. C., Purkis P. E., Leigh I. M., Lane E. B. Keratin 19: predicted amino acid sequence and broad tissue distribution suggest it evolved from keratinocyte keratins. J Invest Dermatol. 1989 May;92(5):707–716. doi: 10.1111/1523-1747.ep12721500. [DOI] [PubMed] [Google Scholar]
- van Muijen G. N., Ruiter D. J., Franke W. W., Achtstätter T., Haasnoot W. H., Ponec M., Warnaar S. O. Cell type heterogeneity of cytokeratin expression in complex epithelia and carcinomas as demonstrated by monoclonal antibodies specific for cytokeratins nos. 4 and 13. Exp Cell Res. 1986 Jan;162(1):97–113. doi: 10.1016/0014-4827(86)90429-5. [DOI] [PubMed] [Google Scholar]
- van Niekerk C. C., Jap P. H., Thomas C. M., Smeets D. F., Ramaekers F. C., Poels L. G. Marker profile of mesothelial cells versus ovarian carcinoma cells. Int J Cancer. 1989 Jun 15;43(6):1065–1071. doi: 10.1002/ijc.2910430619. [DOI] [PubMed] [Google Scholar]
- van de Molengraft F., Ramaekers F., Jap P., Vooijs P., Mungyer G. Changing intermediate-sized filament patterns in metastatic hepatocellular carcinoma cells of the guinea pig. Virchows Arch B Cell Pathol Incl Mol Pathol. 1986;51(4):285–301. doi: 10.1007/BF02899038. [DOI] [PubMed] [Google Scholar]