Abstract
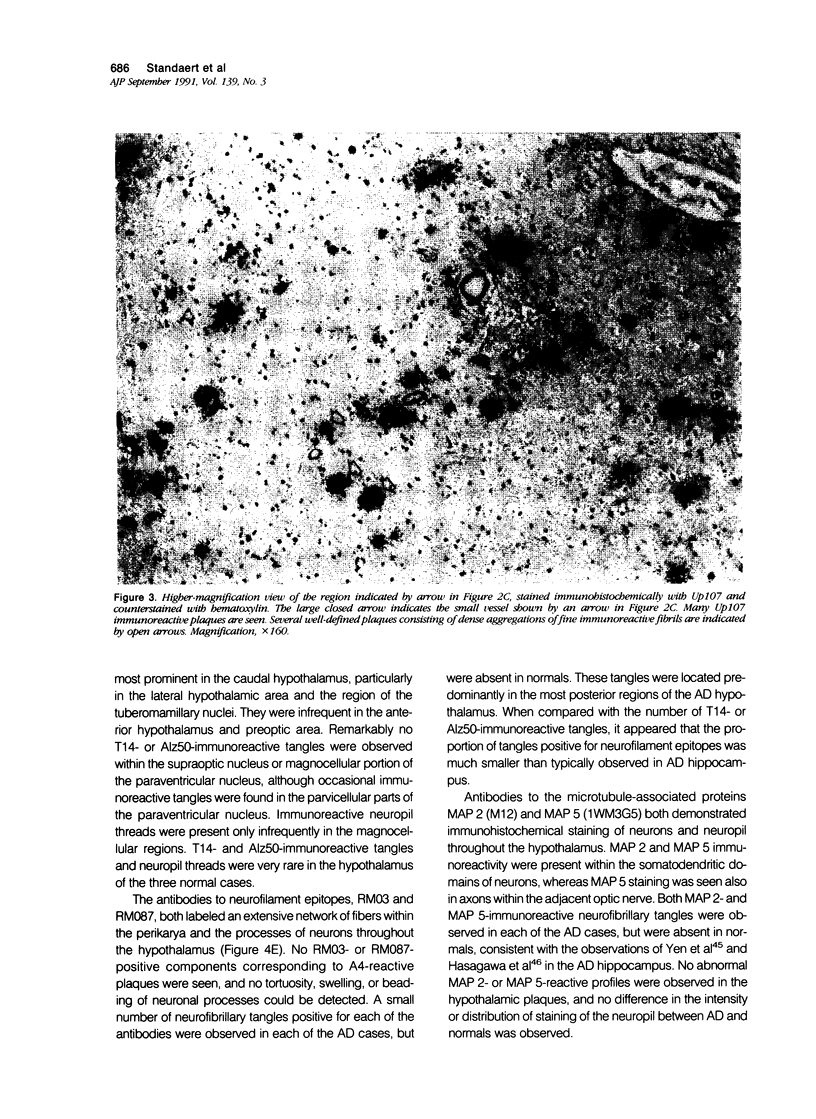
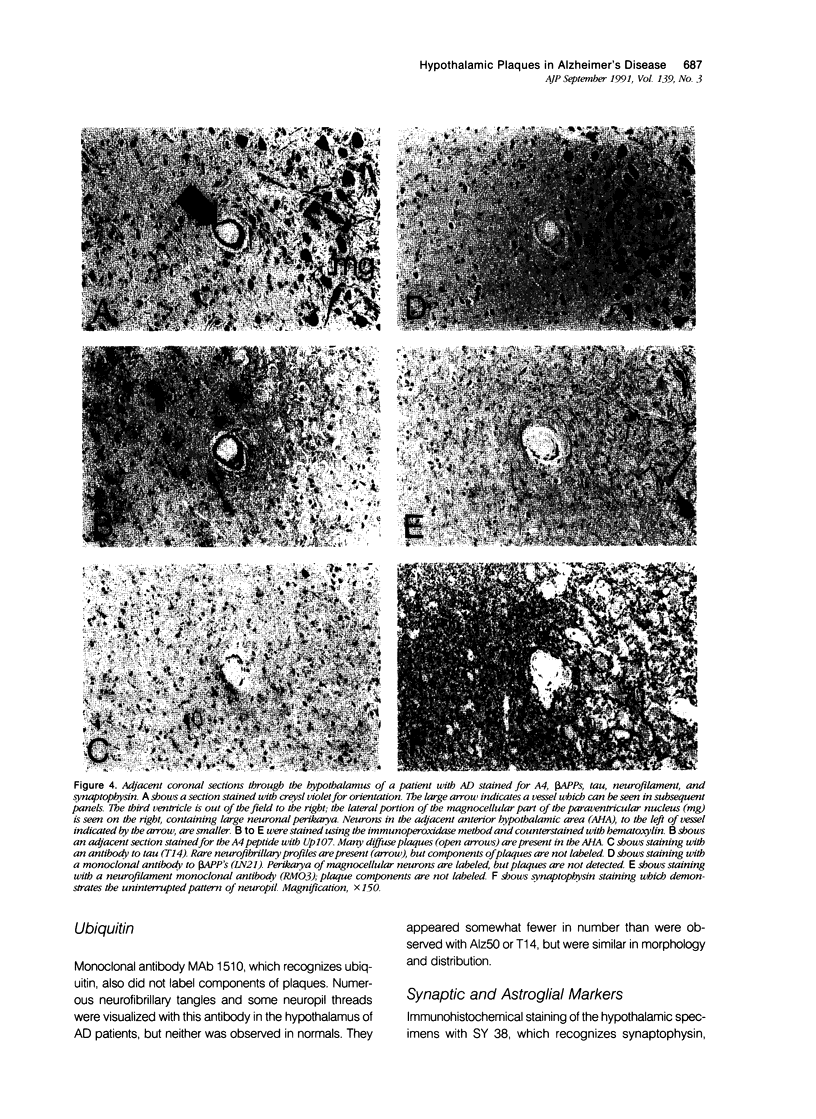
The pathology of Alzheimer's disease (AD) involves subcortical as well as cortical structures. The authors have used immunohistochemical methods to study the molecular composition of AD plaques in the hypothalamus. In contrast to previous studies using histochemical methods, the authors observed large numbers of diffuse plaques in the AD hypothalamus labeled with an antiserum to the beta-amyloid, or A4 peptide, of the beta-amyloid precursor proteins (beta APPs), whereas A4-immunoreactive plaques were uncommon in the hypothalamus of patients without AD. Unlike plaques in the cortex and hippocampus of AD patients, hypothalamic plaques did not contain epitopes corresponding to other regions of the beta APPs, nor did they contain tau-, neurofilament-, or microtubule-associated protein-reactive epitopes, and did not disrupt the neuropil or produce astrogliosis. These findings demonstrate that there are substantial molecular and cellular differences in the pathologic features of AD in the hypothalamus compared with those observed in hippocampal and cortical structures, which may provide insight into the pathogenetic mechanisms of AD.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert S. G., Nakra B. R., Grossberg G. T., Caminal E. R. Vasopressin response to dehydration in Alzheimer's disease. J Am Geriatr Soc. 1989 Sep;37(9):843–847. doi: 10.1111/j.1532-5415.1989.tb02264.x. [DOI] [PubMed] [Google Scholar]
- Arai H., Lee V. M., Otvos L., Jr, Greenberg B. D., Lowery D. E., Sharma S. K., Schmidt M. L., Trojanowski J. Q. Defined neurofilament, tau, and beta-amyloid precursor protein epitopes distinguish Alzheimer from non-Alzheimer senile plaques. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2249–2253. doi: 10.1073/pnas.87.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach T. G., Walker R., McGeer E. G. Patterns of gliosis in Alzheimer's disease and aging cerebrum. Glia. 1989;2(6):420–436. doi: 10.1002/glia.440020605. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M. Clathrin structure characterized with monoclonal antibodies. I. Analysis of multiple antigenic sites. J Cell Biol. 1985 Dec;101(6):2047–2054. doi: 10.1083/jcb.101.6.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark E. A., Leach K. L., Trojanowski J. Q., Lee V. M. Characterization and differential distribution of the three major human protein kinase C isozymes (PKC alpha, PKC beta, and PKC gamma) of the central nervous system in normal and Alzheimer's disease brains. Lab Invest. 1991 Jan;64(1):35–44. [PubMed] [Google Scholar]
- Cras P., Kawai M., Siedlak S., Mulvihill P., Gambetti P., Lowery D., Gonzalez-DeWhitt P., Greenberg B., Perry G. Neuronal and microglial involvement in beta-amyloid protein deposition in Alzheimer's disease. Am J Pathol. 1990 Aug;137(2):241–246. [PMC free article] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984 May 16;120(3):885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Goedert M. Neuronal localization of amyloid beta protein precursor mRNA in normal human brain and in Alzheimer's disease. EMBO J. 1987 Dec 1;6(12):3627–3632. doi: 10.1002/j.1460-2075.1987.tb02694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudsmit E., Hofman M. A., Fliers E., Swaab D. F. The supraoptic and paraventricular nuclei of the human hypothalamus in relation to sex, age and Alzheimer's disease. Neurobiol Aging. 1990 Sep-Oct;11(5):529–536. doi: 10.1016/0197-4580(90)90114-f. [DOI] [PubMed] [Google Scholar]
- Hasegawa M., Arai T., Ihara Y. Immunochemical evidence that fragments of phosphorylated MAP5 (MAP1B) are bound to neurofibrillary tangles in Alzheimer's disease. Neuron. 1990 Jun;4(6):909–918. doi: 10.1016/0896-6273(90)90144-5. [DOI] [PubMed] [Google Scholar]
- Ishii T. Distribution of Alzheimer's neurofibrillary changes in the brain stem and hypothalamus of senile dementia. Acta Neuropathol. 1966 Mar 4;6(2):181–187. doi: 10.1007/BF00686763. [DOI] [PubMed] [Google Scholar]
- Itagaki S., McGeer P. L., Akiyama H., Zhu S., Selkoe D. Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J Neuroimmunol. 1989 Oct;24(3):173–182. doi: 10.1016/0165-5728(89)90115-x. [DOI] [PubMed] [Google Scholar]
- Joachim C. L., Morris J. H., Selkoe D. J. Diffuse senile plaques occur commonly in the cerebellum in Alzheimer's disease. Am J Pathol. 1989 Aug;135(2):309–319. [PMC free article] [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Khachaturian Z. S. Diagnosis of Alzheimer's disease. Arch Neurol. 1985 Nov;42(11):1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- Kitaguchi N., Takahashi Y., Tokushima Y., Shiojiri S., Ito H. Novel precursor of Alzheimer's disease amyloid protein shows protease inhibitory activity. Nature. 1988 Feb 11;331(6156):530–532. doi: 10.1038/331530a0. [DOI] [PubMed] [Google Scholar]
- Kosik K. S., Orecchio L. D., Binder L., Trojanowski J. Q., Lee V. M., Lee G. Epitopes that span the tau molecule are shared with paired helical filaments. Neuron. 1988 Nov;1(9):817–825. doi: 10.1016/0896-6273(88)90129-8. [DOI] [PubMed] [Google Scholar]
- Lee V. M., Balin B. J., Otvos L., Jr, Trojanowski J. Q. A68: a major subunit of paired helical filaments and derivatized forms of normal Tau. Science. 1991 Feb 8;251(4994):675–678. doi: 10.1126/science.1899488. [DOI] [PubMed] [Google Scholar]
- Lee V. M., Carden M. J., Schlaepfer W. W., Trojanowski J. Q. Monoclonal antibodies distinguish several differentially phosphorylated states of the two largest rat neurofilament subunits (NF-H and NF-M) and demonstrate their existence in the normal nervous system of adult rats. J Neurosci. 1987 Nov;7(11):3474–3488. doi: 10.1523/JNEUROSCI.07-11-03474.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V. M., Otvos L., Jr, Carden M. J., Hollosi M., Dietzschold B., Lazzarini R. A. Identification of the major multiphosphorylation site in mammalian neurofilaments. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1998–2002. doi: 10.1073/pnas.85.6.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V. M., Otvos L., Jr, Schmidt M. L., Trojanowski J. Q. Alzheimer disease tangles share immunological similarities with multiphosphorylation repeats in the two large neurofilament proteins. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7384–7388. doi: 10.1073/pnas.85.19.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. A., Higgins G. A., Young W. G., Goldgaber D., Gajdusek D. C., Wilson M. C., Morrison J. H. Distribution of precursor amyloid-beta-protein messenger RNA in human cerebral cortex: relationship to neurofibrillary tangles and neuritic plaques. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1691–1695. doi: 10.1073/pnas.85.5.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E., Terry R. D., Mallory M., Alford M., Hansen L. A. Diffuse plaques do not accentuate synapse loss in Alzheimer's disease. Am J Pathol. 1990 Dec;137(6):1293–1297. [PMC free article] [PubMed] [Google Scholar]
- McDuff T., Sumi S. M. Subcortical degeneration in Alzheimer's disease. Neurology. 1985 Jan;35(1):123–126. doi: 10.1212/wnl.35.1.123. [DOI] [PubMed] [Google Scholar]
- Mendelson W. B. Clinical neuropharmacology of sleep. Neurol Clin. 1990 Feb;8(1):153–160. [PubMed] [Google Scholar]
- Norbiato G., Bevilacqua M., Carella F., Chebat E., Raggi U., Bertora P., Grassi M. P., Mangoni A. Alterations in vasopressin regulation in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1988 Jul;51(7):903–908. doi: 10.1136/jnnp.51.7.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogomori K., Kitamoto T., Tateishi J., Sato Y., Suetsugu M., Abe M. Beta-protein amyloid is widely distributed in the central nervous system of patients with Alzheimer's disease. Am J Pathol. 1989 Feb;134(2):243–251. [PMC free article] [PubMed] [Google Scholar]
- Ponte P., Gonzalez-DeWhitt P., Schilling J., Miller J., Hsu D., Greenberg B., Davis K., Wallace W., Lieberburg I., Fuller F. A new A4 amyloid mRNA contains a domain homologous to serine proteinase inhibitors. Nature. 1988 Feb 11;331(6156):525–527. doi: 10.1038/331525a0. [DOI] [PubMed] [Google Scholar]
- Price D. L., Koo E. H., Unterbeck A. Cellular and molecular biology of Alzheimer's disease. Bioessays. 1989 Feb-Mar;10(2-3):69–74. doi: 10.1002/bies.950100208. [DOI] [PubMed] [Google Scholar]
- Rudelli R. D., Ambler M. W., Wisniewski H. M. Morphology and distribution of Alzheimer neuritic (senile) and amyloid plaques in striatum and diencephalon. Acta Neuropathol. 1984;64(4):273–281. doi: 10.1007/BF00690393. [DOI] [PubMed] [Google Scholar]
- Saper C. B., German D. C. Hypothalamic pathology in Alzheimer's disease. Neurosci Lett. 1987 Mar 9;74(3):364–370. doi: 10.1016/0304-3940(87)90325-9. [DOI] [PubMed] [Google Scholar]
- Schmidt M. L., Lee V. M., Hurtig H., Trojanowski J. Q. Properties of antigenic determinants that distinguish neurofibrillary tangles in progressive supranuclear palsy and Alzheimer's disease. Lab Invest. 1988 Oct;59(4):460–466. [PubMed] [Google Scholar]
- Schmidt M. L., Lee V. M., Trojanowski J. Q. Comparative epitope analysis of neuronal cytoskeletal proteins in Alzheimer's disease senile plaque neurites and neuropil threads. Lab Invest. 1991 Mar;64(3):352–357. [PubMed] [Google Scholar]
- Selkoe D. J., Abraham C. R., Podlisny M. B., Duffy L. K. Isolation of low-molecular-weight proteins from amyloid plaque fibers in Alzheimer's disease. J Neurochem. 1986 Jun;46(6):1820–1834. doi: 10.1111/j.1471-4159.1986.tb08501.x. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. Biochemistry of altered brain proteins in Alzheimer's disease. Annu Rev Neurosci. 1989;12:463–490. doi: 10.1146/annurev.ne.12.030189.002335. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. Molecular pathology of amyloidogenic proteins and the role of vascular amyloidosis in Alzheimer's disease. Neurobiol Aging. 1989 Sep-Oct;10(5):387–395. doi: 10.1016/0197-4580(89)90072-9. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J., Podlisny M. B., Joachim C. L., Vickers E. A., Lee G., Fritz L. C., Oltersdorf T. Beta-amyloid precursor protein of Alzheimer disease occurs as 110- to 135-kilodalton membrane-associated proteins in neural and nonneural tissues. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7341–7345. doi: 10.1073/pnas.85.19.7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G., Chau V. Ubiquitin and microtubule-associated protein tau immunoreactivity each define distinct structures with differing distributions and solubility properties in Alzheimer brain. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2854–2858. doi: 10.1073/pnas.85.8.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson J., Yates C. M., Watts A. G., Fink G. Congo red birefringent structures in the hypothalamus in senile dementia of the Alzheimer type. Neuropathol Appl Neurobiol. 1988 Sep-Oct;14(5):381–393. doi: 10.1111/j.1365-2990.1988.tb01140.x. [DOI] [PubMed] [Google Scholar]
- Stern R. A., Otvos L., Jr, Trojanowski J. Q., Lee V. M. Monoclonal antibodies to a synthetic peptide homologous with the first 28 amino acids of Alzheimer's disease beta-protein recognize amyloid and diverse glial and neuronal cell types in the central nervous system. Am J Pathol. 1989 May;134(5):973–978. [PMC free article] [PubMed] [Google Scholar]
- Suenaga T., Hirano A., Llena J. F., Ksiezak-Reding H., Yen S. H., Dickson D. W. Modified Bielschowsky and immunocytochemical studies on cerebellar plaques in Alzheimer's disease. J Neuropathol Exp Neurol. 1990 Jan;49(1):31–40. doi: 10.1097/00005072-199001000-00004. [DOI] [PubMed] [Google Scholar]
- Tagliavini F., Giaccone G., Frangione B., Bugiani O. Preamyloid deposits in the cerebral cortex of patients with Alzheimer's disease and nondemented individuals. Neurosci Lett. 1988 Nov 11;93(2-3):191–196. doi: 10.1016/0304-3940(88)90080-8. [DOI] [PubMed] [Google Scholar]
- Tanzi R. E., Gusella J. F., Watkins P. C., Bruns G. A., St George-Hyslop P., Van Keuren M. L., Patterson D., Pagan S., Kurnit D. M., Neve R. L. Amyloid beta protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science. 1987 Feb 20;235(4791):880–884. doi: 10.1126/science.2949367. [DOI] [PubMed] [Google Scholar]
- Tanzi R. E., McClatchey A. I., Lamperti E. D., Villa-Komaroff L., Gusella J. F., Neve R. L. Protease inhibitor domain encoded by an amyloid protein precursor mRNA associated with Alzheimer's disease. Nature. 1988 Feb 11;331(6156):528–530. doi: 10.1038/331528a0. [DOI] [PubMed] [Google Scholar]
- Thomas R., Williams P., John R., Scanlon M. Growth hormone responses to growth hormone releasing factor in primary degenerative dementia. Biol Psychiatry. 1989 Aug;26(4):389–396. doi: 10.1016/0006-3223(89)90055-3. [DOI] [PubMed] [Google Scholar]
- Trojanowski J. Q., Schmidt M. L., Otvos L., Jr, Arai H., Hill W. D., Lee V. M. Vulnerability of the neuronal cytoskeleton in aging and Alzheimer disease: widespread involvement of all three major filament systems. Annu Rev Gerontol Geriatr. 1990;10:167–182. doi: 10.1007/978-3-662-38445-9_10. [DOI] [PubMed] [Google Scholar]
- Trojanowski J. Q., Schuck T., Schmidt M. L., Lee V. M. Distribution of phosphate-independent MAP2 epitopes revealed with monoclonal antibodies in microwave-denatured human nervous system tissues. J Neurosci Methods. 1989 Aug;29(2):171–180. doi: 10.1016/0165-0270(89)90030-7. [DOI] [PubMed] [Google Scholar]
- Trojanowski J. Q., Schuck T., Schmidt M. L., Lee V. M. Distribution of tau proteins in the normal human central and peripheral nervous system. J Histochem Cytochem. 1989 Feb;37(2):209–215. doi: 10.1177/37.2.2492045. [DOI] [PubMed] [Google Scholar]
- Ulrich J. Alzheimer changes in nondemented patients younger than sixty-five: possible early stages of Alzheimer's disease and senile dementia of Alzheimer type. Ann Neurol. 1985 Mar;17(3):273–277. doi: 10.1002/ana.410170309. [DOI] [PubMed] [Google Scholar]
- Vogels O. J., Broere C. A., Nieuwenhuys R. Neuronal hypertrophy in the human supraoptic and paraventricular nucleus in aging and Alzheimer's disease. Neurosci Lett. 1990 Feb 5;109(1-2):62–67. doi: 10.1016/0304-3940(90)90538-k. [DOI] [PubMed] [Google Scholar]
- Wiedenmann B., Franke W. W. Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell. 1985 Jul;41(3):1017–1028. doi: 10.1016/s0092-8674(85)80082-9. [DOI] [PubMed] [Google Scholar]
- Witting W., Kwa I. H., Eikelenboom P., Mirmiran M., Swaab D. F. Alterations in the circadian rest-activity rhythm in aging and Alzheimer's disease. Biol Psychiatry. 1990 Mar 15;27(6):563–572. doi: 10.1016/0006-3223(90)90523-5. [DOI] [PubMed] [Google Scholar]
- Wolozin B. L., Pruchnicki A., Dickson D. W., Davies P. A neuronal antigen in the brains of Alzheimer patients. Science. 1986 May 2;232(4750):648–650. doi: 10.1126/science.3083509. [DOI] [PubMed] [Google Scholar]
- Wong D. H., Ignatius M. J., Parosky G., Parham P., Trojanowski J. Q., Brodsky F. M. Neuron-specific expression of high-molecular-weight clathrin light chain. J Neurosci. 1990 Sep;10(9):3025–3031. doi: 10.1523/JNEUROSCI.10-09-03025.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H., Hirai S., Morimatsu M., Shoji M., Ihara Y. A variety of cerebral amyloid deposits in the brains of the Alzheimer-type dementia demonstrated by beta protein immunostaining. Acta Neuropathol. 1988;76(6):541–549. doi: 10.1007/BF00689591. [DOI] [PubMed] [Google Scholar]
- Yen S. H., Dickson D. W., Crowe A., Butler M., Shelanski M. L. Alzheimer's neurofibrillary tangles contain unique epitopes and epitopes in common with the heat-stable microtubule associated proteins tau and MAP2. Am J Pathol. 1987 Jan;126(1):81–91. [PMC free article] [PubMed] [Google Scholar]