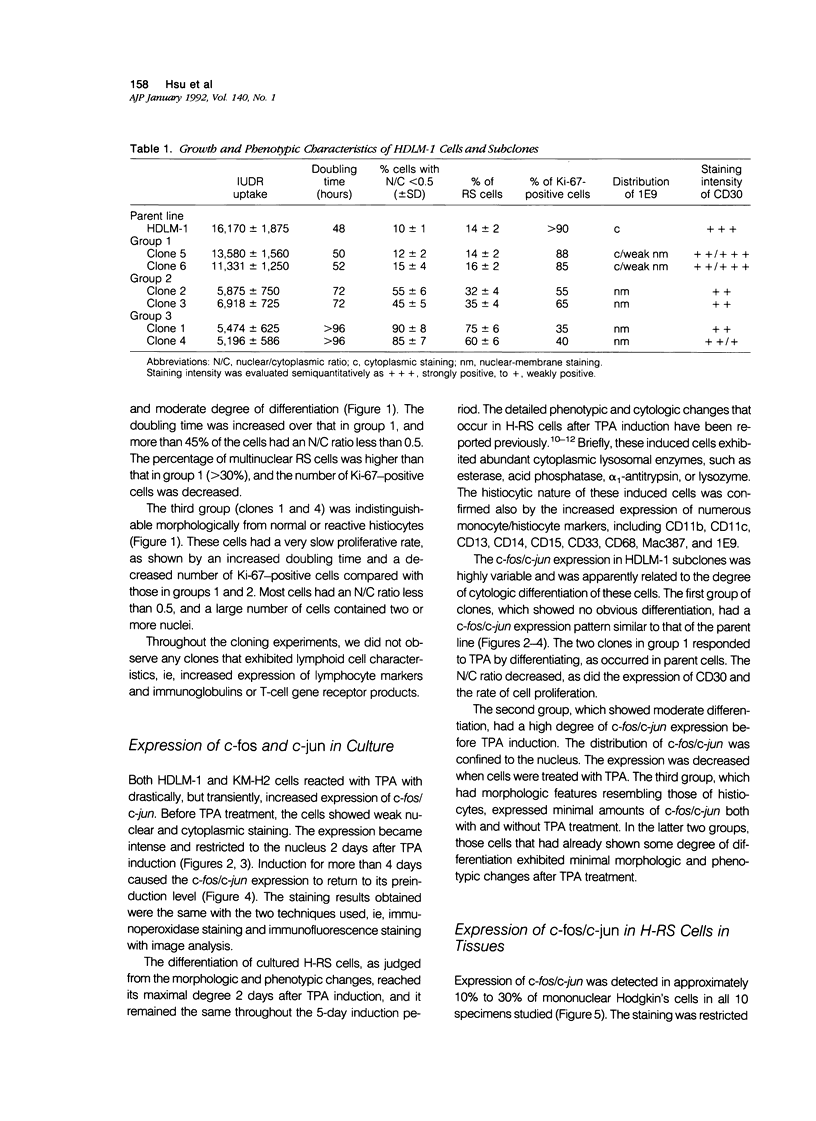
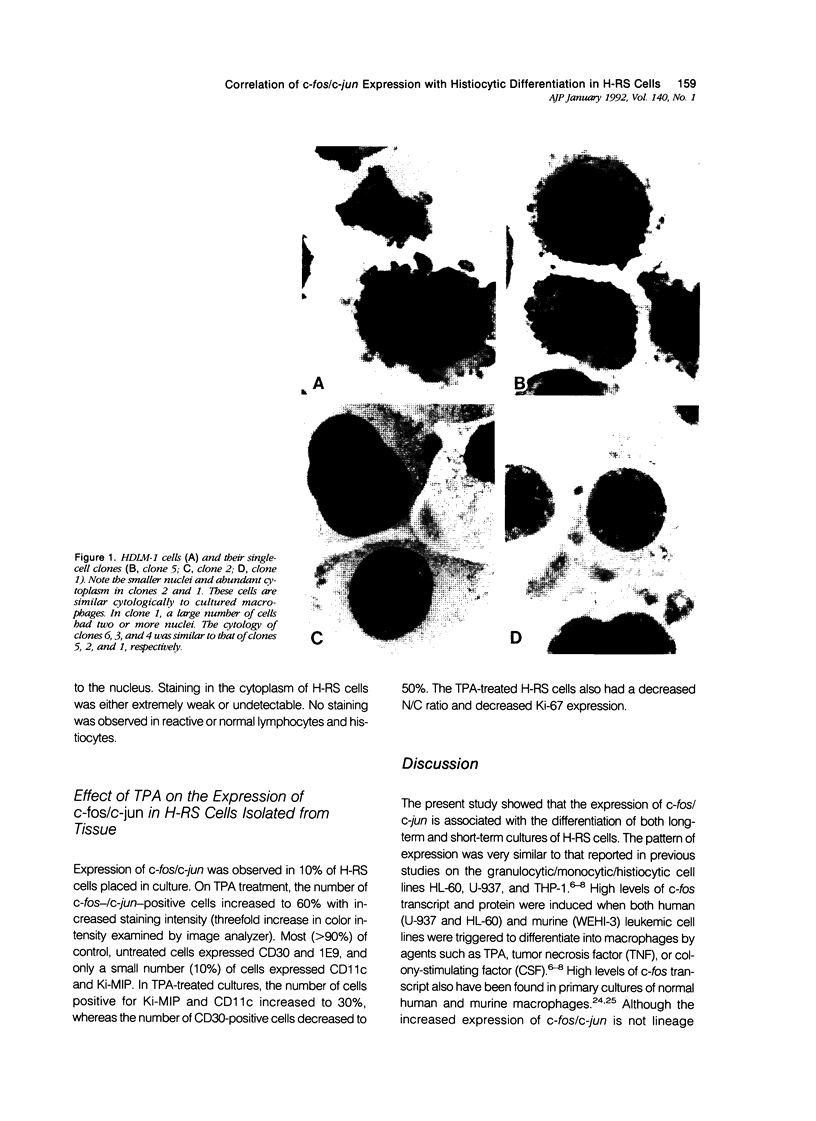
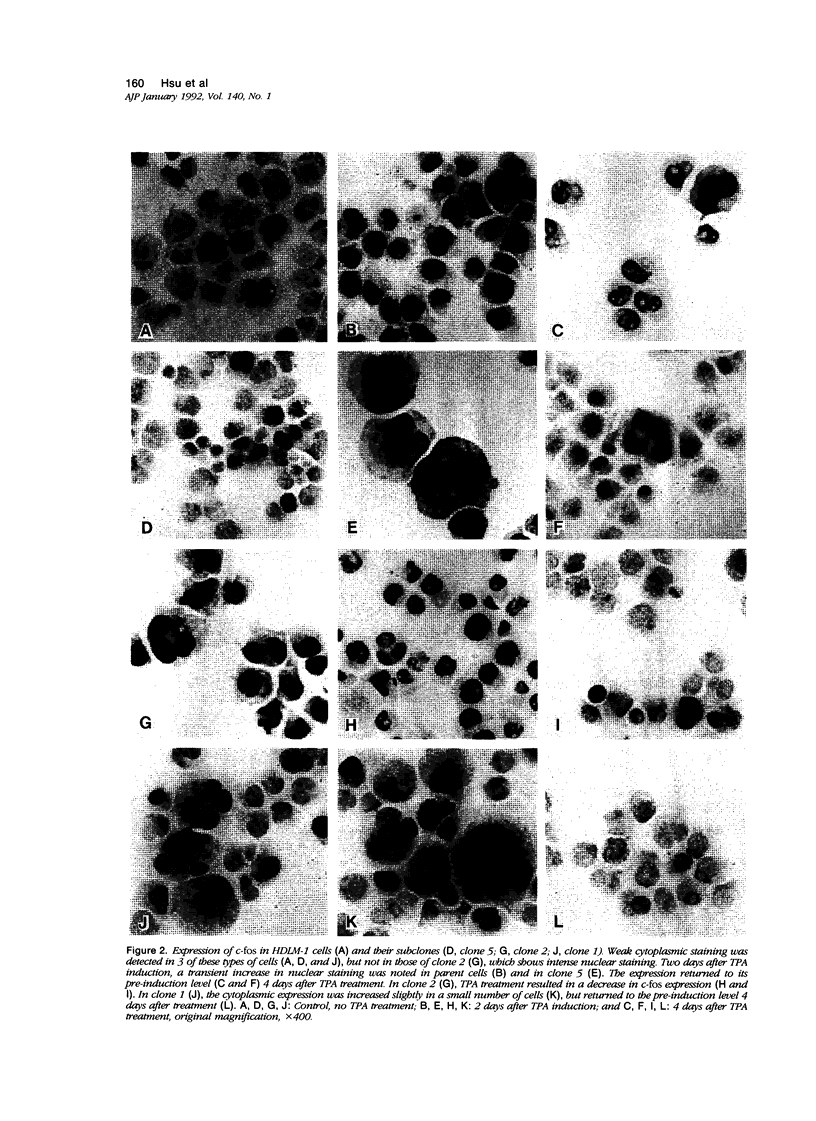
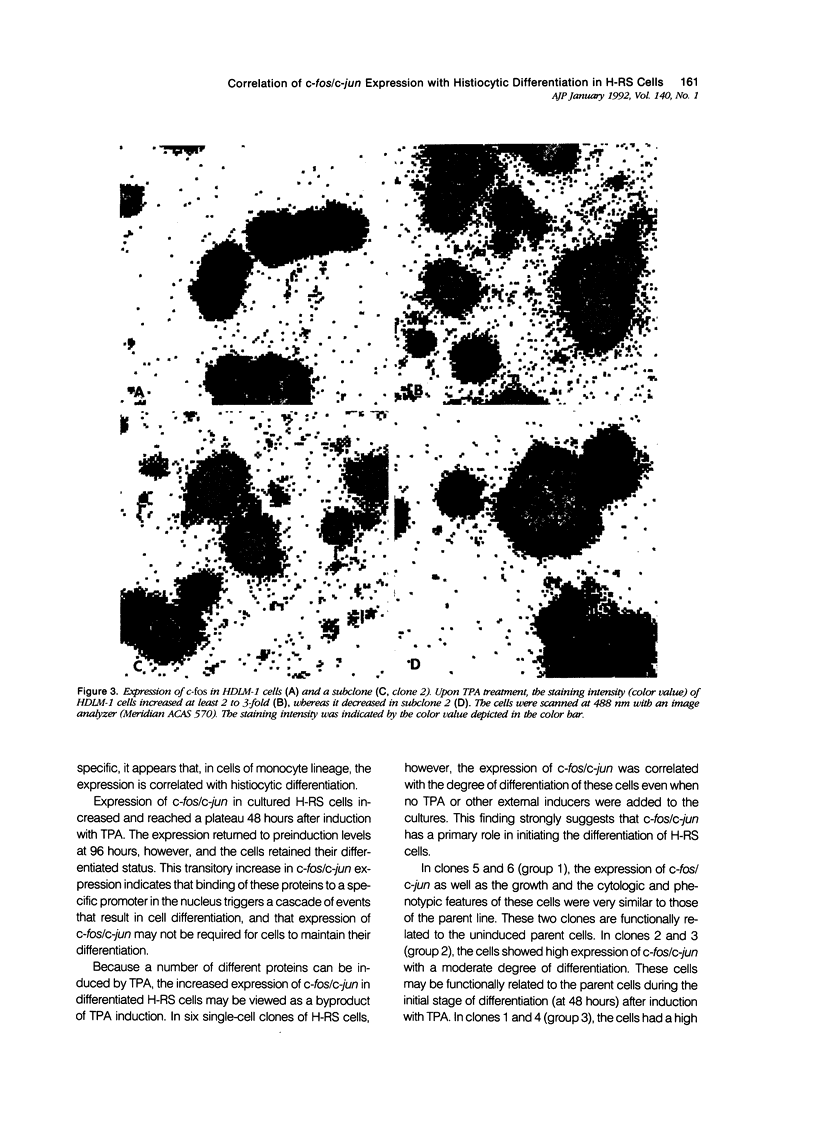
Abstract
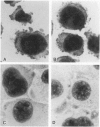
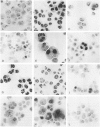
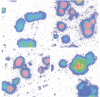
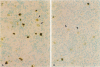
The c-fos proto-oncogene, which is the normal homolog of the transforming gene carried by murine osteogenic sarcoma viruses, interacts with the protein product of another proto-oncogene, c-jun, to form a heterodimer that can recognize and bind to a specific sequence of nucleotides in the DNA. The expression of c-fos and c-jun is linked to the proliferation of certain cells and the differentiation of others, including those of monomyelocyte lineage. The authors used two cultured Hodgkin's Reed-Sternberg (H-RS) cell lines, KM-H2 and HDLM-1, and their single-cell clones to study the correlation of c-fos/c-jun expression with cell differentiation in H-RS cells. Within 48 hours after induction with phorbol ester (TPA), both parent lines exhibited markedly increased expression of c-fos/c-jun. The expression returned to the preinduction level after 96 hours, however, and the cells retained their differentiated status. The transitory increase in c-fos/c-jun expression suggests that binding of these proteins to a specific promoter in the nucleus triggers a cascade of events that result in cell differentiation. Expression of these proteins may not be required for the cells to maintain their differentiation. The authors selected three groups of sublines of HDLM-1 cells based on their degree of spontaneous cytologic differentiation. The first group, without obvious differentiation, showed a c-fos/c-jun expression pattern similar to that of the parent line. The second group, with moderate differentiation, had a high degree of expression, which decreased on treatment with TPA. The third group, which had morphologic features resembling those of histiocytes, expressed minimal amounts of c-fos/c-jun, irrespective of TPA treatment. These findings provide further evidence that c-fos/c-jun expression is related to differentiation of H-RS cells, and that these proteins are not byproducts of TPA induction. Expression of c-fos/c-jun also was noted in a subpopulation of H-RS cells in tissues; and this expression also was enhanced when these cells were treated with TPA in culture. These findings indicate that H-RS cells can differentiate to become mature-appearing cells in tissues.
Full text
PDF


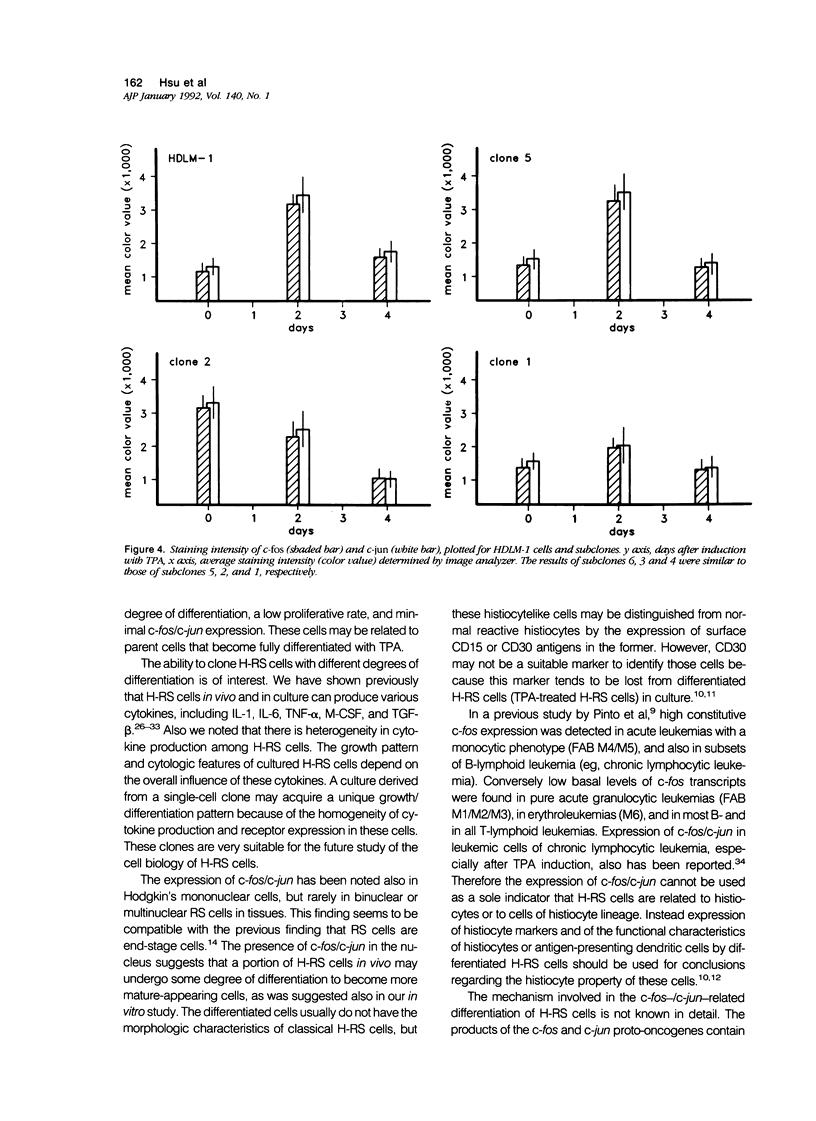
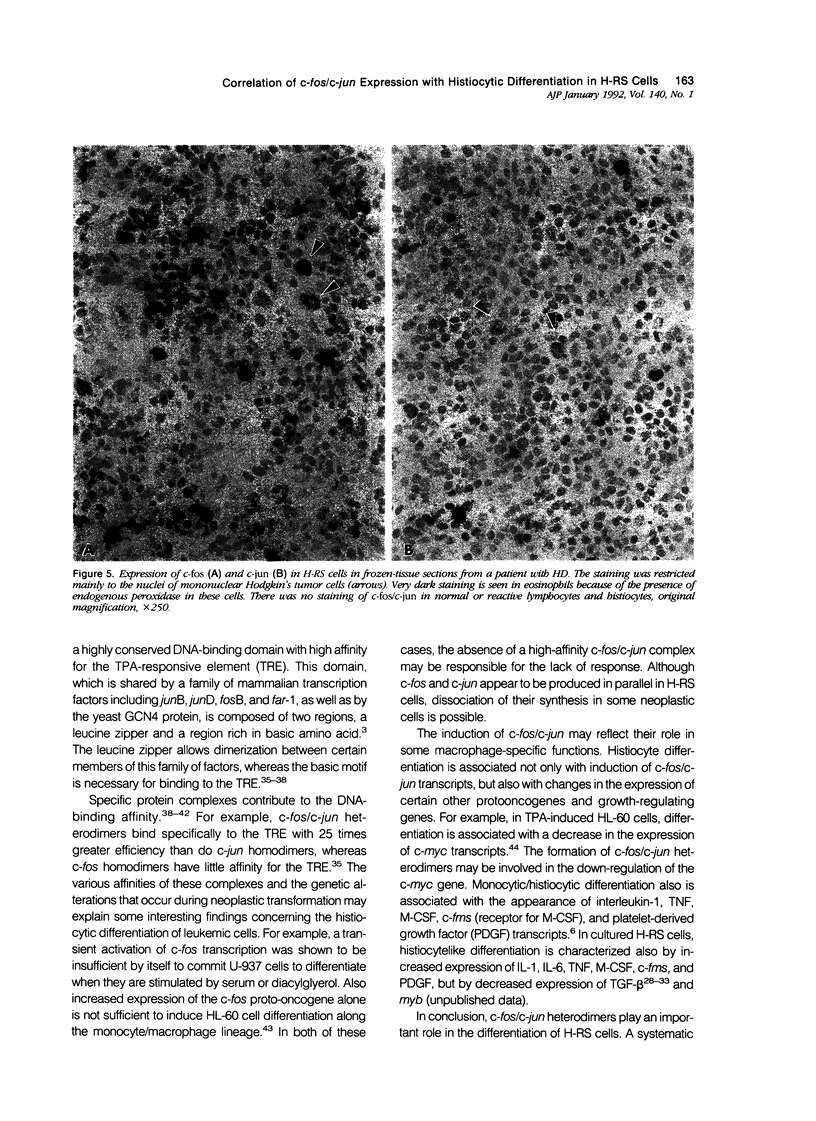


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brach M. A., Riedel D., Herrmann F. Induction of monocytic differentiation and modulation of the expression of c-fos, c-fms and c-myc protooncogenes in human monoblasts by cytokines and phorbolester. Virchows Arch B Cell Pathol Incl Mol Pathol. 1990;59(1):54–58. doi: 10.1007/BF02899387. [DOI] [PubMed] [Google Scholar]
- Curran T., Franza B. R., Jr Fos and Jun: the AP-1 connection. Cell. 1988 Nov 4;55(3):395–397. doi: 10.1016/0092-8674(88)90024-4. [DOI] [PubMed] [Google Scholar]
- Drexler H. G., Gaedicke G., Lok M. S., Diehl V., Minowada J. Hodgkin's disease derived cell lines HDLM-2 and L-428: comparison of morphology, immunological and isoenzyme profiles. Leuk Res. 1986;10(5):487–500. doi: 10.1016/0145-2126(86)90084-6. [DOI] [PubMed] [Google Scholar]
- Drexler H. G., Janssen J. W., Brenner M. K., Hoffbrand A. V., Bartram C. R. Rapid expression of protooncogenes c-fos and c-myc in B-chronic lymphocytic leukemia cells during differentiation induced by phorbol ester and calcium ionophore. Blood. 1989 May 1;73(6):1656–1663. [PubMed] [Google Scholar]
- Gentz R., Rauscher F. J., 3rd, Abate C., Curran T. Parallel association of Fos and Jun leucine zippers juxtaposes DNA binding domains. Science. 1989 Mar 31;243(4899):1695–1699. doi: 10.1126/science.2494702. [DOI] [PubMed] [Google Scholar]
- Halazonetis T. D., Georgopoulos K., Greenberg M. E., Leder P. c-Jun dimerizes with itself and with c-Fos, forming complexes of different DNA binding affinities. Cell. 1988 Dec 2;55(5):917–924. doi: 10.1016/0092-8674(88)90147-x. [DOI] [PubMed] [Google Scholar]
- Hecht T. T., Longo D. L., Cossman J., Bolen J. B., Hsu S. M., Israel M., Fisher R. I. Production and characterization of a monoclonal antibody that binds Reed-Sternberg cells. J Immunol. 1985 Jun;134(6):4231–4236. [PubMed] [Google Scholar]
- Ho Y. S., Lee W. M., Snyderman R. Chemoattractant-induced activation of c-fos gene expression in human monocytes. J Exp Med. 1987 Jun 1;165(6):1524–1538. doi: 10.1084/jem.165.6.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu P. L., Hsu S. M. Identification of an Mr 70,000 antigen associated with Reed-Sternberg cells and interdigitating reticulum cells. Cancer Res. 1990 Jan 15;50(2):350–357. [PubMed] [Google Scholar]
- Hsu P. L., Hsu S. M. Production of tumor necrosis factor-alpha and lymphotoxin by cells of Hodgkin's neoplastic cell lines HDLM-1 and KM-H2. Am J Pathol. 1989 Oct;135(4):735–745. [PMC free article] [PubMed] [Google Scholar]
- Hsu P. L., Lin Y. C., Hsu S. M. Expression of macrophage colony-stimulating factor (M-CSF) in two Hodgkin's Reed-Sternberg (H-RS) cell lines, HDLM-1 and KM-H2, and in H-RS cells in tissues. Int J Hematol. 1991 Aug;54(4):315–326. [PubMed] [Google Scholar]
- Hsu S. M., Hsu P. L. Phenotypes and phorbol ester-induced differentiation of human histiocytic lymphoma cell lines (U-937 and SU-DHL-1) and Reed-Sternberg cells. Am J Pathol. 1986 Feb;122(2):223–230. [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Pescovitz M. D., Hsu P. L. Monoclonal antibodies against SU-DHL-1 cells stain the neoplastic cells in true histiocytic lymphoma, malignant histiocytosis, and Hodgkin's disease. Blood. 1986 Jul;68(1):213–219. [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Soban E. Color modification of diaminobenzidine (DAB) precipitation by metallic ions and its application for double immunohistochemistry. J Histochem Cytochem. 1982 Oct;30(10):1079–1082. doi: 10.1177/30.10.6182185. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Xie S. S., Hsu P. L. Cultured Reed-Sternberg cells HDLM-1 and KM-H2 can be induced to become histiocytelike cells. H-RS cells are not derived from lymphocytes. Am J Pathol. 1990 Aug;137(2):353–367. [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Yang K., Jaffe E. S. Phenotypic expression of Hodgkin's and Reed-Sternberg cells in Hodgkin's disease. Am J Pathol. 1985 Feb;118(2):209–217. [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Zhao X., Chakraborty S., Liu Y. F., Whang-Peng J., Lok M. S., Fukuhara S. Reed-Sternberg cells in Hodgkin's cell lines HDLM, L-428, and KM-H2 are not actively replicating: lack of bromodeoxyuridine uptake by multinuclear cells in culture. Blood. 1988 May;71(5):1382–1389. [PubMed] [Google Scholar]
- Hsu S. M., Zhao X. Expression of interleukin-1 in Reed-Sternberg cells and neoplastic cells from true histiocytic malignancies. Am J Pathol. 1986 Nov;125(2):221–225. [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Zhao X., Hsu P. L., Lok M. S. Extracellular matrix does not induce the proliferation, but promotes the differentiation, of Hodgkin's cell line HDLM-1. Am J Pathol. 1987 Apr;127(1):9–14. [PMC free article] [PubMed] [Google Scholar]
- Kamesaki H., Fukuhara S., Tatsumi E., Uchino H., Yamabe H., Miwa H., Shirakawa S., Hatanaka M., Honjo T. Cytochemical, immunologic, chromosomal, and molecular genetic analysis of a novel cell line derived from Hodgkin's disease. Blood. 1986 Jul;68(1):285–292. [PubMed] [Google Scholar]
- Kouzarides T., Ziff E. Leucine zippers of fos, jun and GCN4 dictate dimerization specificity and thereby control DNA binding. Nature. 1989 Aug 17;340(6234):568–571. doi: 10.1038/340568a0. [DOI] [PubMed] [Google Scholar]
- Kouzarides T., Ziff E. The role of the leucine zipper in the fos-jun interaction. Nature. 1988 Dec 15;336(6200):646–651. doi: 10.1038/336646a0. [DOI] [PubMed] [Google Scholar]
- Kruijer W., Schubert D., Verma I. M. Induction of the proto-oncogene fos by nerve growth factor. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7330–7334. doi: 10.1073/pnas.82.21.7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Mitchell R. L., Henning-Chubb C., Huberman E., Verma I. M. c-fos expression is neither sufficient nor obligatory for differentiation of monomyelocytes to macrophages. Cell. 1986 May 23;45(4):497–504. doi: 10.1016/0092-8674(86)90281-3. [DOI] [PubMed] [Google Scholar]
- Mitchell R. L., Zokas L., Schreiber R. D., Verma I. M. Rapid induction of the expression of proto-oncogene fos during human monocytic differentiation. Cell. 1985 Jan;40(1):209–217. doi: 10.1016/0092-8674(85)90324-1. [DOI] [PubMed] [Google Scholar]
- Nakabeppu Y., Ryder K., Nathans D. DNA binding activities of three murine Jun proteins: stimulation by Fos. Cell. 1988 Dec 2;55(5):907–915. doi: 10.1016/0092-8674(88)90146-8. [DOI] [PubMed] [Google Scholar]
- Pinto A., Colletta G., Del Vecchio L., Rosati R., Attadia V., Cimino R., Colombatti A. c-fos oncogene expression in human hematopoietic malignancies is restricted to acute leukemias with monocytic phenotype and to subsets of B cell leukemias. Blood. 1987 Nov;70(5):1450–1457. [PubMed] [Google Scholar]
- Sassone-Corsi P., Ransone L. J., Lamph W. W., Verma I. M. Direct interaction between fos and jun nuclear oncoproteins: role of the 'leucine zipper' domain. Nature. 1988 Dec 15;336(6200):692–695. doi: 10.1038/336692a0. [DOI] [PubMed] [Google Scholar]
- Schwab U., Stein H., Gerdes J., Lemke H., Kirchner H., Schaadt M., Diehl V. Production of a monoclonal antibody specific for Hodgkin and Sternberg-Reed cells of Hodgkin's disease and a subset of normal lymphoid cells. Nature. 1982 Sep 2;299(5878):65–67. doi: 10.1038/299065a0. [DOI] [PubMed] [Google Scholar]
- Sherman M. L., Stone R. M., Datta R., Bernstein S. H., Kufe D. W. Transcriptional and post-transcriptional regulation of c-jun expression during monocytic differentiation of human myeloid leukemic cells. J Biol Chem. 1990 Feb 25;265(6):3320–3323. [PubMed] [Google Scholar]
- Squinto S. P., Doucet J. P., Block A. L., Morrow S. L., Davenport W. D., Jr Induction of macrophage-like differentiation of HL-60 leukemia cells by tumor necrosis factor-alpha: potential role of fos expression. Mol Endocrinol. 1989 Feb;3(2):409–419. doi: 10.1210/mend-3-2-409. [DOI] [PubMed] [Google Scholar]
- Stone R. M., Weber B. L., Spriggs D. R., Kufe D. W. Phospholipase C activates protein kinase C and induces monocytic differentiation of HL-60 cells. Blood. 1988 Aug;72(2):739–744. [PubMed] [Google Scholar]
- Turner R., Tjian R. Leucine repeats and an adjacent DNA binding domain mediate the formation of functional cFos-cJun heterodimers. Science. 1989 Mar 31;243(4899):1689–1694. doi: 10.1126/science.2494701. [DOI] [PubMed] [Google Scholar]
- Willman C. L., Fenoglio-Preiser C. M. Oncogenes, suppressor genes, and carcinogenesis. Hum Pathol. 1987 Sep;18(9):895–902. doi: 10.1016/s0046-8177(87)80266-6. [DOI] [PubMed] [Google Scholar]
- Zerial M., Toschi L., Ryseck R. P., Schuermann M., Müller R., Bravo R. The product of a novel growth factor activated gene, fos B, interacts with JUN proteins enhancing their DNA binding activity. EMBO J. 1989 Mar;8(3):805–813. doi: 10.1002/j.1460-2075.1989.tb03441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]