Abstract
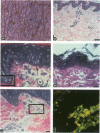
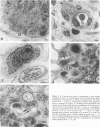
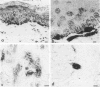
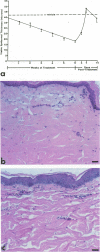
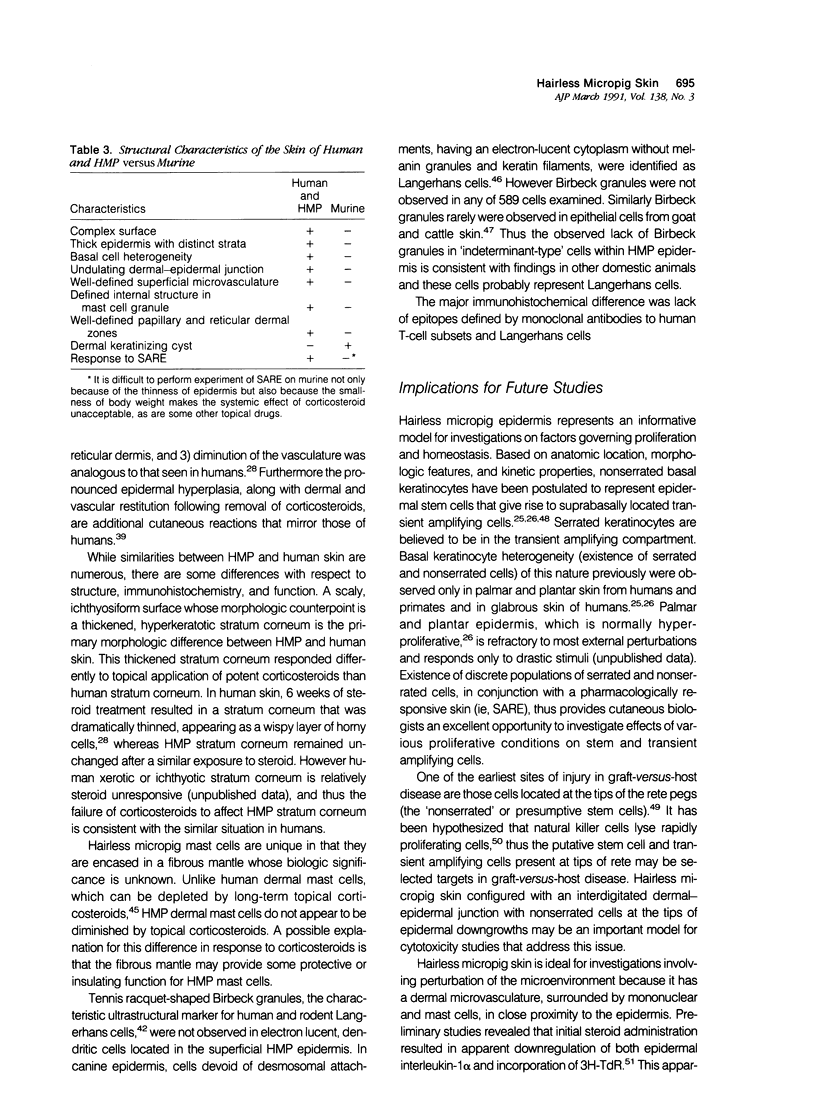
Reported here is the structural and immunohistochemical similarities between the Yucatan hairless micropig (HMP) skin and that of humans. Hairless micropig skin surface was composed of complex intersecting furrows that created geometric patterns remarkably similar to human skin surface glyphics. The dermal--epidermal interface consisted of undulant downgrowths that interdigitated with dermal papillae. Hairless micropig epidermis contained two morphologically distinct populations of basal keratinocytes (serrated and nonserrated). Similar heterogeneity has been seen only in human epidermis and primate palmar epidermis. Immunohistochemistry revealed that the HMP epidermis is reactive with monoclonal and polyclonal antisera to keratin proteins. Melanocytes reactive with antisera to S-100 protein, as in human skin, also were observed in HMP epidermis. Organization of dermal extracellular matrix, including collagen and elastic fibers, and the organization and reactivity of the microvasculature with antisera to factor VIII, were consistent with human skin. The costicosteroid-induced atrophy and subsequent rebound phenomenon after withdrawal of steroid observed in HMP skin was similar with that observed in humans. It is concluded that HMP skin approximates human skin significantly more precisely than most existing species and is an excellent model for studies of cutaneous physiology and pharmacology.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belsito D. V., Flotte T. J., Lim H. W., Baer R. L., Thorbecke G. J., Gigli I. Effect of glucocorticosteroids on epidermal Langerhans cells. J Exp Med. 1982 Jan 1;155(1):291–302. doi: 10.1084/jem.155.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braverman I. M. Ultrastructure and organization of the cutaneous microvasculature in normal and pathologic states. J Invest Dermatol. 1989 Aug;93(2 Suppl):2S–9S. doi: 10.1111/1523-1747.ep12580893. [DOI] [PubMed] [Google Scholar]
- Cocchia D., Michetti F., Donato R. Immunochemical and immuno-cytochemical localization of S-100 antigen in normal human skin. Nature. 1981 Nov 5;294(5836):85–87. doi: 10.1038/294085a0. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G., Cheng S. Z., Dong G., Sun T. T., Lavker R. M. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989 Apr 21;57(2):201–209. doi: 10.1016/0092-8674(89)90958-6. [DOI] [PubMed] [Google Scholar]
- Dale B. A., Lonsdale-Eccles J. D., Holbrook K. A. Stratum corneum basic protein: an interfilamentous matrix protein of epidermal keratin. Curr Probl Dermatol. 1980;10:311–325. doi: 10.1159/000396298. [DOI] [PubMed] [Google Scholar]
- Elias P. M., Fritsch P. O., Lampe M., Williams M. L., Brown B. E., Nemanic M., Grayson S. Retinoid effects on epidermal structure, differentiation, and permeability. Lab Invest. 1981 Jun;44(6):531–540. [PubMed] [Google Scholar]
- Flotte T. J., Springer T. A., Thorbecke G. J. Dendritic cell and macrophage staining by monoclonal antibodies in tissue sections and epidermal sheets. Am J Pathol. 1983 Apr;111(1):112–124. [PMC free article] [PubMed] [Google Scholar]
- Green H. Terminal differentiation of cultured human epidermal cells. Cell. 1977 Jun;11(2):405–416. doi: 10.1016/0092-8674(77)90058-7. [DOI] [PubMed] [Google Scholar]
- Guillén F. J., Ferrara J., Hancock W. W., Messadi D., Fonferko E., Burakoff S. J., Murphy G. F. Acute cutaneous graft-versus-host disease to minor histocompatibility antigens in a murine model. Evidence that large granular lymphocytes are effector cells in the immune response. Lab Invest. 1986 Jul;55(1):35–42. [PubMed] [Google Scholar]
- HIGGINBOTHAM R. D., DOUGHERTY T. F., JEE W. S. Fate of shed mast cell granules. Proc Soc Exp Biol Med. 1956 Jun;92(2):256–261. doi: 10.3181/00379727-92-22445. [DOI] [PubMed] [Google Scholar]
- Higgins J. C., Eady R. A. Human dermal microvasculature: I. Its segmental differentiation. Light and electron microscopic study. Br J Dermatol. 1981 Feb;104(2):117–129. doi: 10.1111/j.1365-2133.1981.tb00033.x. [DOI] [PubMed] [Google Scholar]
- Hintner H., Fritsch P. O., Foidart J. M., Stingl G., Schuler G., Katz S. I. Expression of basement membrane zone antigens at the dermo-epibolic junction in organ cultures of human skin. J Invest Dermatol. 1980 Apr;74(4):200–204. doi: 10.1111/1523-1747.ep12541715. [DOI] [PubMed] [Google Scholar]
- Janossy G., Tidman N., Selby W. S., Thomas J. A., Granger S., Kung P. C., Goldstein G. Human T lymphocytes of inducer and suppressor type occupy different microenvironments. Nature. 1980 Nov 6;288(5786):81–84. doi: 10.1038/288081a0. [DOI] [PubMed] [Google Scholar]
- Karasek M. A. Culture of human keratinocytes in liquid medium. J Invest Dermatol. 1983 Jul;81(1 Suppl):24s–28s. doi: 10.1111/1523-1747.ep12540284. [DOI] [PubMed] [Google Scholar]
- Katz S. I., Tamaki K., Sachs D. H. Epidermal Langerhans cells are derived from cells originating in bone marrow. Nature. 1979 Nov 15;282(5736):324–326. doi: 10.1038/282324a0. [DOI] [PubMed] [Google Scholar]
- Kligman L. H., Akin F. J., Kligman A. M. The contributions of UVA and UVB to connective tissue damage in hairless mice. J Invest Dermatol. 1985 Apr;84(4):272–276. doi: 10.1111/1523-1747.ep12265353. [DOI] [PubMed] [Google Scholar]
- Kligman L. H., Kligman A. M. The effect on rhino mouse skin of agents which influence keratinization and exfoliation. J Invest Dermatol. 1979 Nov;73(5):354–358. doi: 10.1111/1523-1747.ep12550409. [DOI] [PubMed] [Google Scholar]
- Kligman L. H. Luna's technique. A beautiful stain for elastin. Am J Dermatopathol. 1981 Summer;3(2):199–201. doi: 10.1097/00000372-198100320-00014. [DOI] [PubMed] [Google Scholar]
- Kubilus J. Modulation of differentiation by retinoids. J Invest Dermatol. 1983 Jul;81(1 Suppl):55s–58s. doi: 10.1111/1523-1747.ep12540570. [DOI] [PubMed] [Google Scholar]
- Lavker R. M., Grove G. L., Kligman A. M. The atrophogenic effect of crude coal tar on human epidermis. Br J Dermatol. 1981 Jul;105(1):77–82. doi: 10.1111/j.1365-2133.1981.tb00885.x. [DOI] [PubMed] [Google Scholar]
- Lavker R. M., Kligman A. M. Chronic heliodermatitis: a morphologic evaluation of chronic actinic dermal damage with emphasis on the role of mast cells. J Invest Dermatol. 1988 Mar;90(3):325–330. doi: 10.1111/1523-1747.ep12456193. [DOI] [PubMed] [Google Scholar]
- Lavker R. M., Kwong F., Kligman A. M. Changes in skin surface patterns with age. J Gerontol. 1980 May;35(3):348–354. doi: 10.1093/geronj/35.3.348. [DOI] [PubMed] [Google Scholar]
- Lavker R. M., Matoltsy A. G. Substructure of keratohyalin granules of the epidermis as revealed by high resolution electron microscopy. J Ultrastruct Res. 1971 Jun;35(5):575–581. doi: 10.1016/s0022-5320(71)80012-6. [DOI] [PubMed] [Google Scholar]
- Lavker R. M., Schechter N. M. Cutaneous mast cell depletion results from topical corticosteroid usage. J Immunol. 1985 Oct;135(4):2368–2373. [PubMed] [Google Scholar]
- Lavker R. M., Sun T. T. Epidermal stem cells. J Invest Dermatol. 1983 Jul;81(1 Suppl):121s–127s. doi: 10.1111/1523-1747.ep12540880. [DOI] [PubMed] [Google Scholar]
- Lavker R. M., Sun T. T. Heterogeneity in epidermal basal keratinocytes: morphological and functional correlations. Science. 1982 Mar 5;215(4537):1239–1241. doi: 10.1126/science.7058342. [DOI] [PubMed] [Google Scholar]
- Lavker R. M., Sun T. T. Rapid modulation of keratinocyte differentiation by the external environment. J Invest Dermatol. 1983 Apr;80(4):228–237. doi: 10.1111/1523-1747.ep12534527. [DOI] [PubMed] [Google Scholar]
- Lehmann P., Zheng P., Lavker R. M., Kligman A. M. Corticosteroid atrophy in human skin. A study by light, scanning, and transmission electron microscopy. J Invest Dermatol. 1983 Aug;81(2):169–176. doi: 10.1111/1523-1747.ep12543603. [DOI] [PubMed] [Google Scholar]
- MATOLTSY A. G., PARAKKAL P. F. MEMBRANE-COATING GRANULES OF KERATINIZING EPITHELIA. J Cell Biol. 1965 Feb;24:297–307. doi: 10.1083/jcb.24.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONTAGNA W., CHASE H. B., MELARAGNO H. P. The skin of hairless mice. I. The formation of cysts and the distribution of lipids. J Invest Dermatol. 1952 Jul;19(1):83–94. doi: 10.1038/jid.1952.67. [DOI] [PubMed] [Google Scholar]
- Mackenzie I. C. Ordered structure of the epidermis. J Invest Dermatol. 1975 Jul;65(1):45–51. doi: 10.1111/1523-1747.ep12598037. [DOI] [PubMed] [Google Scholar]
- Matoltsy A. G., Matoltsy M. N. The membrane protein of horny cells. J Invest Dermatol. 1966 Jan;46(1):127–129. [PubMed] [Google Scholar]
- Metcalfe D. D., Kaliner M., Donlon M. A. The mast cell. Crit Rev Immunol. 1981 Sep;3(1):23–74. [PubMed] [Google Scholar]
- Mukai K., Rosai J., Burgdorf W. H. Localization of factor VIII-related antigen in vascular endothelial cells using an immunoperoxidase method. Am J Surg Pathol. 1980 Jun;4(3):273–276. doi: 10.1097/00000478-198006000-00008. [DOI] [PubMed] [Google Scholar]
- Murphy G. F., Flynn T. C., Rice R. H., Pinkus G. S. Involucrin expression in normal and neoplastic human skin: a marker for keratinocyte differentiation. J Invest Dermatol. 1984 May;82(5):453–457. doi: 10.1111/1523-1747.ep12260945. [DOI] [PubMed] [Google Scholar]
- Rice R. H., Green H. The cornified envelope of terminally differentiated human epidermal keratinocytes consists of cross-linked protein. Cell. 1977 Jun;11(2):417–422. doi: 10.1016/0092-8674(77)90059-9. [DOI] [PubMed] [Google Scholar]
- Ringler D. J., Hancock W. W., King N. W., Murphy G. F. Characterization of nonhuman primate epidermal and dermal dendritic cells with monoclonal antibodies. A study of Langerhans cells and indeterminate cells in the rhesus monkey. Lab Invest. 1987 Mar;56(3):313–320. [PubMed] [Google Scholar]
- Rowden G., Phillips T. M., Lewis M. G. Ia antigens on indeterminate cells of the epidermis: immunoelectronmicroscopic studies of surface antigens. Br J Dermatol. 1979 May;100(5):531–542. doi: 10.1111/j.1365-2133.1979.tb05578.x. [DOI] [PubMed] [Google Scholar]
- SCHWARTZMAN R. M. THE REACTION OF CANINE SKIN TO HISTAMINE AND 48/80. J Invest Dermatol. 1965 Jan;44:39–42. [PubMed] [Google Scholar]
- Sale G. E., Shulman H. M., Gallucci B. B., Thomas E. D. Young rete ridge keratinocytes are preferred targets in cutaneous graft-versus-host disease. Am J Pathol. 1985 Feb;118(2):278–287. [PMC free article] [PubMed] [Google Scholar]
- Schechter N. M., Choi J. K., Slavin D. A., Deresienski D. T., Sayama S., Dong G., Lavker R. M., Proud D., Lazarus G. S. Identification of a chymotrypsin-like proteinase in human mast cells. J Immunol. 1986 Aug 1;137(3):962–970. [PubMed] [Google Scholar]
- Stegman S. J. A study of dermabrasion and chemical peels in an animal model. J Dermatol Surg Oncol. 1980 Jun;6(6):490–497. doi: 10.1111/j.1524-4725.1980.tb00903.x. [DOI] [PubMed] [Google Scholar]
- Tsao M. C., Walthall B. J., Ham R. G. Clonal growth of normal human epidermal keratinocytes in a defined medium. J Cell Physiol. 1982 Feb;110(2):219–229. doi: 10.1002/jcp.1041100217. [DOI] [PubMed] [Google Scholar]
- Zheng P. S., Lavker R. M., Lehmann P., Kligman A. M. Morphologic investigations on the rebound phenomenon after corticosteroid-induced atrophy in human skin. J Invest Dermatol. 1984 Apr;82(4):345–352. doi: 10.1111/1523-1747.ep12260665. [DOI] [PubMed] [Google Scholar]
- du Boulay C. E. Demonstration of alpha-1-antitrypsin and alpha-1-antichymotrypsin in fibrous histiocytomas using the immunoperoxidase technique. Am J Surg Pathol. 1982 Sep;6(6):559–564. doi: 10.1097/00000478-198209000-00008. [DOI] [PubMed] [Google Scholar]