Abstract
All-trans retinoic acid (RA) has beneficial effects when used in a variety of inflammatory skin conditions. In this study, the authors found that RA inhibited superoxide anion production and proteolytic enzyme release by human and rat neutrophils. Concomitantly, the authors found that RA-treated neutrophils were less able than untreated neutrophils to injure endothelial cells in culture even though the adhesion of the RA-treated neutrophils to endothelial cell monolayers was not diminished. Inhibition of cytotoxicity occurred over the same range of concentrations that inhibited oxygen radical formation and protease release. In additional studies, it was observed that pretreatment of endothelial cells with RA-induced resistance to subsequent injury by activated neutrophils. Finally, in vivo studies showed that pretreatment of rats for 3 days with RA (1-10 mg/day, IP) reduced the degree of injury in the lungs and skin sites after treatment with bovine serum albumin and antibodies to bovine serum albumin in the reverse-passive Arthus reaction. Thus, RA can modulate neutrophil-mediated endothelial cell injury by an effect on both the neutrophils and their target cells. Together, these effects may underlie the reduction in immune complex-mediated injury seen in experimental animals. The beneficial effects that retinoids have in a variety of inflammatory skin diseases may likewise be a reflection of their effects on the physiology of both neutrophils and endothelial cells.
Full text
PDF


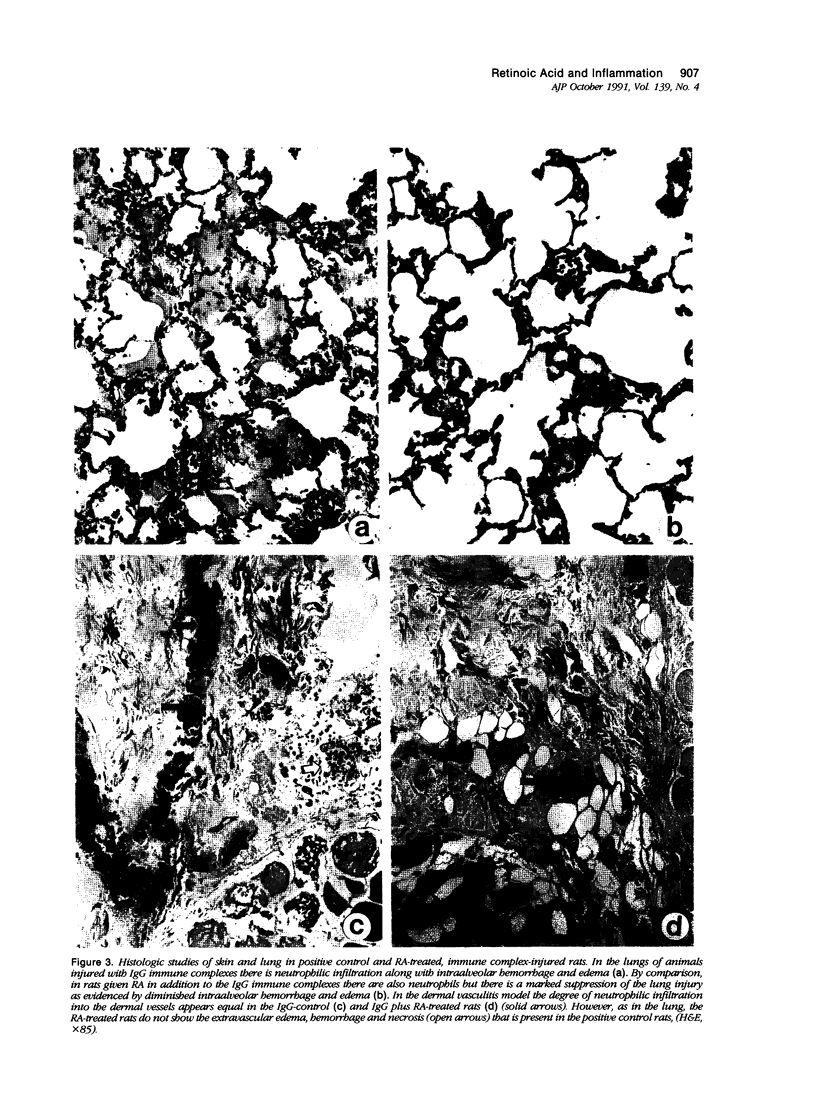


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badwey J. A., Horn W., Heyworth P. G., Robinson J. M., Karnovsky M. L. Paradoxical effects of retinal in neutrophil stimulation. J Biol Chem. 1989 Sep 5;264(25):14947–14953. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Camisa C., Eisenstat B., Ragaz A., Weissmann G. The effects of retinoids on neutrophil functions in vitro. J Am Acad Dermatol. 1982 Apr;6(4 Pt 2 Suppl):620–629. doi: 10.1016/s0190-9622(82)70051-9. [DOI] [PubMed] [Google Scholar]
- Chytil F., Sherman D. R. How do retinoids work? Dermatologica. 1987;175 (Suppl 1):8–12. doi: 10.1159/000248847. [DOI] [PubMed] [Google Scholar]
- Dicken C. H. Retinoids: a review. J Am Acad Dermatol. 1984 Oct;11(4 Pt 1):541–552. doi: 10.1016/s0190-9622(84)70204-0. [DOI] [PubMed] [Google Scholar]
- Dubertret L., Lebreton C., Touraine R. Inhibition of neutrophil migration by etretinate and its main metabolite. Br J Dermatol. 1982 Dec;107(6):681–685. doi: 10.1111/j.1365-2133.1982.tb00528.x. [DOI] [PubMed] [Google Scholar]
- Gannon D. E., He X. M., Ward P. A., Varani J., Johnson K. J. Time-dependent inhibition of oxygen radical induced lung injury. Inflammation. 1990 Oct;14(5):509–522. doi: 10.1007/BF00914272. [DOI] [PubMed] [Google Scholar]
- Gannon D. E., Varani J., Phan S. H., Ward J. H., Kaplan J., Till G. O., Simon R. H., Ryan U. S., Ward P. A. Source of iron in neutrophil-mediated killing of endothelial cells. Lab Invest. 1987 Jul;57(1):37–44. [PubMed] [Google Scholar]
- Ginsburg I., Gibbs D. F., Schuger L., Johnson K. J., Ryan U. S., Ward P. A., Varani J. Vascular endothelial cell killing by combinations of membrane-active agents and hydrogen peroxide. Free Radic Biol Med. 1989;7(4):369–376. doi: 10.1016/0891-5849(89)90123-8. [DOI] [PubMed] [Google Scholar]
- Ginsburg I., Ward P. A., Varani J. Lysophosphatides enhance superoxide responses of stimulated human neutrophils. Inflammation. 1989 Apr;13(2):163–174. doi: 10.1007/BF00924787. [DOI] [PubMed] [Google Scholar]
- Goldstein J. A., Comite H., Mescon H., Pochi P. E. Isotretinoin in the treatment of acne: histologic changes, sebum production, and clinical observations. Arch Dermatol. 1982 Aug;118(8):555–558. [PubMed] [Google Scholar]
- Harms M. Traitement de l'acné par l'isotrétinoïne par voie buccale. Etude clinique sur 56 patients. Schweiz Med Wochenschr. 1983 Oct 22;113(42):1549–1554. [PubMed] [Google Scholar]
- Johnson K. J., Ward P. A. Role of oxygen metabolites in immune complex injury of lung. J Immunol. 1981 Jun;126(6):2365–2369. [PubMed] [Google Scholar]
- Kensler T. W., Trush M. A. Inhibiton of phorbol ester-stimulated chemiluminescence in human polymorphonuclear leukocytes by retinoic acid and 5,6-epoxyretinoic acid. Cancer Res. 1981 Jan;41(1):216–222. [PubMed] [Google Scholar]
- Kurtz P. J., Emmerling D. C., Donofrio D. J. Subchronic toxicity of all-trans-retinoic acid and retinylidene dimedone in Sprague-Dawley rats. Toxicology. 1984 Mar;30(2):115–124. doi: 10.1016/0300-483x(84)90122-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Markey B. A., Phan S. H., Varani J., Ryan U. S., Ward P. A. Inhibition of cytotoxicity by intracellular superoxide dismutase supplementation. Free Radic Biol Med. 1990;9(4):307–314. doi: 10.1016/0891-5849(90)90005-4. [DOI] [PubMed] [Google Scholar]
- Meeks R. G., Zaharevitz D., Chen R. F. Membrane effects of retinoids: possible correlation with toxicity. Arch Biochem Biophys. 1981 Mar;207(1):141–147. doi: 10.1016/0003-9861(81)90019-9. [DOI] [PubMed] [Google Scholar]
- Norris D. A., Osborn R., Robinson W., Tonnesen M. G. Isotretinoin produces significant inhibition of monocyte and neutrophil chemotaxis in vivo in patients with cystic acne. J Invest Dermatol. 1987 Jul;89(1):38–43. doi: 10.1111/1523-1747.ep12580370. [DOI] [PubMed] [Google Scholar]
- Oldham K. T., Guice K. S., Ward P. A., Johnson K. J. The role of oxygen radicals in immune complex injury. Free Radic Biol Med. 1988;4(6):387–397. doi: 10.1016/0891-5849(88)90090-1. [DOI] [PubMed] [Google Scholar]
- Phan S. H., Gannon D. E., Varani J., Ryan U. S., Ward P. A. Xanthine oxidase activity in rat pulmonary artery endothelial cells and its alteration by activated neutrophils. Am J Pathol. 1989 Jun;134(6):1201–1211. [PMC free article] [PubMed] [Google Scholar]
- Pigatto P. D., Fioroni A., Riva F., Brugo M. A., Morandotti A., Altomare G. F., Finzi A. F. Effects of isotretinoin on the neutrophil chemotaxis in cystic acne. Dermatologica. 1983;167(1):16–18. doi: 10.1159/000249738. [DOI] [PubMed] [Google Scholar]
- Plewig G., Wagner A. Anti-inflammatory effects of 13-Cis-retinoic acid. An in vivo study. Arch Dermatol Res. 1981;270(1):89–94. doi: 10.1007/BF00417154. [DOI] [PubMed] [Google Scholar]
- Schuger L., Varani J., Marks R. M., Kunkel S. L., Johnson K. J., Ward P. A. Cytotoxicity of tumor necrosis factor-alpha for human umbilical vein endothelial cells. Lab Invest. 1989 Jul;61(1):62–68. [PubMed] [Google Scholar]
- Varani J., Fligiel S. E., Till G. O., Kunkel R. G., Ryan U. S., Ward P. A. Pulmonary endothelial cell killing by human neutrophils. Possible involvement of hydroxyl radical. Lab Invest. 1985 Dec;53(6):656–663. [PubMed] [Google Scholar]
- Varani J., Ginsburg I., Schuger L., Gibbs D. F., Bromberg J., Johnson K. J., Ryan U. S., Ward P. A. Endothelial cell killing by neutrophils. Synergistic interaction of oxygen products and proteases. Am J Pathol. 1989 Sep;135(3):435–438. [PMC free article] [PubMed] [Google Scholar]
- Varani J., Phan S. H., Gibbs D. F., Ryan U. S., Ward P. A. H2O2-mediated cytotoxicity of rat pulmonary endothelial cells. Changes in adenosine triphosphate and purine products and effects of protective interventions. Lab Invest. 1990 Nov;63(5):683–689. [PubMed] [Google Scholar]
- Varani J., Ward D., Johnson K. J. Nonspecific protease and elastase activities in rat leukocytes. Inflammation. 1982 Jun;6(2):177–187. doi: 10.1007/BF00916242. [DOI] [PubMed] [Google Scholar]
- Wolfson M., Shinwell E. S., Zvillich M., Rager-Zisman B. Inhibitory effect of retinoic acid on the respiratory burst of adult and cord blood neutrophils and macrophages: potential implication to bronchopulmonary dysplasia. Clin Exp Immunol. 1988 Jun;72(3):505–509. [PMC free article] [PubMed] [Google Scholar]
- Yoshioka A., Miyachi Y., Imamura S., Niwa Y. Anti-oxidant effects of retinoids on inflammatory skin diseases. Arch Dermatol Res. 1986;278(3):177–183. doi: 10.1007/BF00412920. [DOI] [PubMed] [Google Scholar]
- van de Kerkhof P. C., Chang A., van Dooren-Greebe R., Geiger J. M., Happle R. Intra-epidermal accumulation of polymorphonuclear leukocytes in persistent palmoplantar pustulosis during treatment with acitretin. Acta Derm Venereol. 1988;68(6):499–503. [PubMed] [Google Scholar]