Abstract
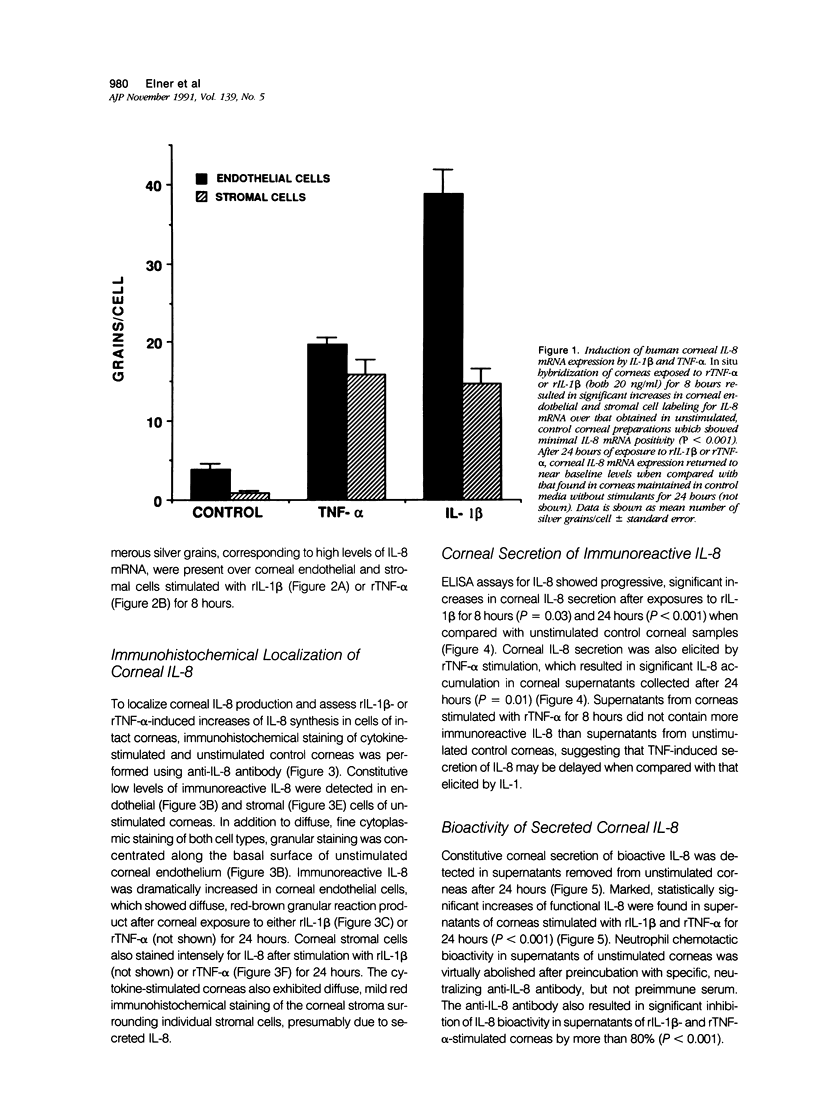
Corneal leukocytic infiltration is an important component of numerous ocular diseases, but specific corneal-derived leukocyte chemotaxins have not been identified. In this study, the authors identified interleukin-8 (IL-8), a known neutrophil and lymphocyte chemotaxin, to be an important chemotaxin produced by human corneal tissue. In situ hybridization and immunohistochemistry of corneas exposed to human recombinant (r) interleukin-1-beta (rIL-1 beta) or tumor necrosis factor-alpha (rTNF-alpha) revealed significant increases in corneal endothelial and stromal cell IL-8 mRNA (P less than 0.001) and marked increases in cell-associated immunoreactive IL-8 compared with unstimulated controls. ELISA assays revealed four- to eight-fold increases in corneal IL-8 secretion after 24-hour exposures to either cytokine over that obtained with unstimulated corneas (P = 0.01). In neutrophil chemotactic bioassays, significant increases in functional IL-8 were detected in media conditioned by corneas exposed to rIL-1 beta or rTNF-alpha for 24 hours (P less than 0.001). Preincubation of these corneal media with anti-IL-8 antibody significantly reduced neutrophil chemotaxis by more than 80%. These results suggest that the cornea is an active participant in ocular inflammation and raise the possibility that agents used in experimental corneal pocket models may produce indirect effects by inducing corneal secretion of other factors, such as IL-8.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akira S., Hirano T., Taga T., Kishimoto T. Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF). FASEB J. 1990 Aug;4(11):2860–2867. [PubMed] [Google Scholar]
- Barker J. N., Sarma V., Mitra R. S., Dixit V. M., Nickoloff B. J. Marked synergism between tumor necrosis factor-alpha and interferon-gamma in regulation of keratinocyte-derived adhesion molecules and chemotactic factors. J Clin Invest. 1990 Feb;85(2):605–608. doi: 10.1172/JCI114481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BenEzra D., Hemo I., Maftzir G. In vivo angiogenic activity of interleukins. Arch Ophthalmol. 1990 Apr;108(4):573–576. doi: 10.1001/archopht.1990.01070060121061. [DOI] [PubMed] [Google Scholar]
- Colditz I., Zwahlen R., Dewald B., Baggiolini M. In vivo inflammatory activity of neutrophil-activating factor, a novel chemotactic peptide derived from human monocytes. Am J Pathol. 1989 Apr;134(4):755–760. [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Elgebaly S. A., Herkert N., O'Rourke J., Kreutzer D. L. Characterization of neutrophil and monocyte specific chemotactic factors derived from the cornea in response to hydrogen peroxide injury. Am J Pathol. 1987 Jan;126(1):40–50. [PMC free article] [PubMed] [Google Scholar]
- Elner V. M., Elner S. G., Pavilack M. A., Todd R. F., 3rd, Yue B. Y., Huber A. R. Intercellular adhesion molecule-1 in human corneal endothelium. Modulation and function. Am J Pathol. 1991 Mar;138(3):525–536. [PMC free article] [PubMed] [Google Scholar]
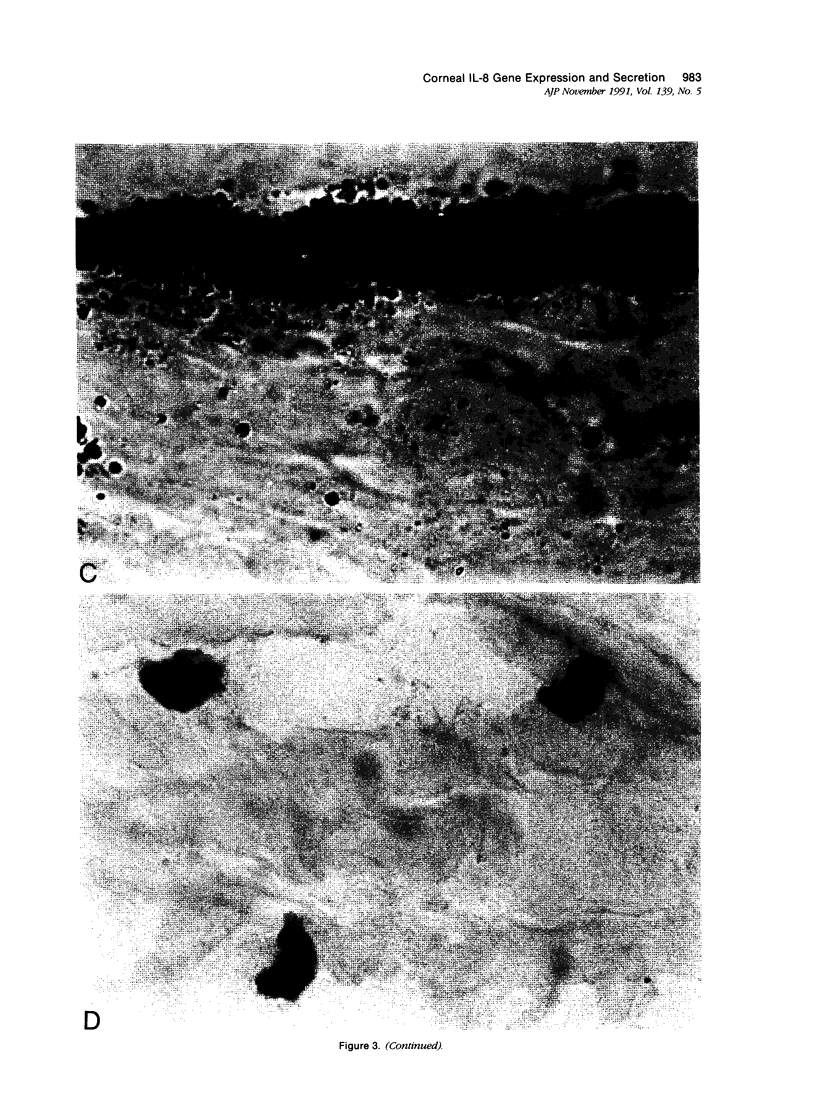
- Harvath L., Falk W., Leonard E. J. Rapid quantitation of neutrophil chemotaxis: use of a polyvinylpyrrolidone-free polycarbonate membrane in a multiwell assembly. J Immunol Methods. 1980;37(1):39–45. doi: 10.1016/0022-1759(80)90179-9. [DOI] [PubMed] [Google Scholar]
- Kunkel S. L., Spengler M., May M. A., Spengler R., Larrick J., Remick D. Prostaglandin E2 regulates macrophage-derived tumor necrosis factor gene expression. J Biol Chem. 1988 Apr 15;263(11):5380–5384. [PubMed] [Google Scholar]
- Larsen C. G., Anderson A. O., Appella E., Oppenheim J. J., Matsushima K. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science. 1989 Mar 17;243(4897):1464–1466. doi: 10.1126/science.2648569. [DOI] [PubMed] [Google Scholar]
- Larsen C. G., Zachariae C. O., Oppenheim J. J., Matsushima K. Production of monocyte chemotactic and activating factor (MCAF) by human dermal fibroblasts in response to interleukin 1 or tumor necrosis factor. Biochem Biophys Res Commun. 1989 May 15;160(3):1403–1408. doi: 10.1016/s0006-291x(89)80160-3. [DOI] [PubMed] [Google Scholar]
- Le J. M., Weinstein D., Gubler U., Vilcek J. Induction of membrane-associated interleukin 1 by tumor necrosis factor in human fibroblasts. J Immunol. 1987 Apr 1;138(7):2137–2142. [PubMed] [Google Scholar]
- Leibovich S. J., Polverini P. J., Shepard H. M., Wiseman D. M., Shively V., Nuseir N. Macrophage-induced angiogenesis is mediated by tumour necrosis factor-alpha. Nature. 1987 Oct 15;329(6140):630–632. doi: 10.1038/329630a0. [DOI] [PubMed] [Google Scholar]
- Libby P., Ordovas J. M., Auger K. R., Robbins A. H., Birinyi L. K., Dinarello C. A. Endotoxin and tumor necrosis factor induce interleukin-1 gene expression in adult human vascular endothelial cells. Am J Pathol. 1986 Aug;124(2):179–185. [PMC free article] [PubMed] [Google Scholar]
- Luscinskas F. W., Brock A. F., Arnaout M. A., Gimbrone M. A., Jr Endothelial-leukocyte adhesion molecule-1-dependent and leukocyte (CD11/CD18)-dependent mechanisms contribute to polymorphonuclear leukocyte adhesion to cytokine-activated human vascular endothelium. J Immunol. 1989 Apr 1;142(7):2257–2263. [PubMed] [Google Scholar]
- Matsushima K., Larsen C. G., DuBois G. C., Oppenheim J. J. Purification and characterization of a novel monocyte chemotactic and activating factor produced by a human myelomonocytic cell line. J Exp Med. 1989 Apr 1;169(4):1485–1490. doi: 10.1084/jem.169.4.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima K., Morishita K., Yoshimura T., Lavu S., Kobayashi Y., Lew W., Appella E., Kung H. F., Leonard E. J., Oppenheim J. J. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumor necrosis factor. J Exp Med. 1988 Jun 1;167(6):1883–1893. doi: 10.1084/jem.167.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima K., Oppenheim J. J. Interleukin 8 and MCAF: novel inflammatory cytokines inducible by IL 1 and TNF. Cytokine. 1989 Nov;1(1):2–13. doi: 10.1016/1043-4666(89)91043-0. [DOI] [PubMed] [Google Scholar]
- Osborn L., Hession C., Tizard R., Vassallo C., Luhowskyj S., Chi-Rosso G., Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989 Dec 22;59(6):1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- Ponte P., Ng S. Y., Engel J., Gunning P., Kedes L. Evolutionary conservation in the untranslated regions of actin mRNAs: DNA sequence of a human beta-actin cDNA. Nucleic Acids Res. 1984 Feb 10;12(3):1687–1696. doi: 10.1093/nar/12.3.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley M. V., Elgebaly S. A. The release of a neutrophil chemotactic factor from UV-B irradiated rabbit corneas in vitro. Curr Eye Res. 1990 Jul;9(7):677–682. doi: 10.3109/02713689008999583. [DOI] [PubMed] [Google Scholar]
- Rosenbaum J. T., Howes E. L., Jr, Rubin R. M., Samples J. R. Ocular inflammatory effects of intravitreally-injected tumor necrosis factor. Am J Pathol. 1988 Oct;133(1):47–53. [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum J. T., Samples J. R., Hefeneider S. H., Howes E. L., Jr Ocular inflammatory effects of intravitreal interleukin 1. Arch Ophthalmol. 1987 Aug;105(8):1117–1120. doi: 10.1001/archopht.1987.01060080119040. [DOI] [PubMed] [Google Scholar]
- Shams N. B., Sigel M. M., Davis R. M. Interferon-gamma, Staphylococcus aureus, and lipopolysaccharide/silica enhance interleukin-1 beta production by human corneal cells. Reg Immunol. 1989 May-Jun;2(3):136–148. [PubMed] [Google Scholar]
- Strieter R. M., Kunkel S. L., Showell H. J., Remick D. G., Phan S. H., Ward P. A., Marks R. M. Endothelial cell gene expression of a neutrophil chemotactic factor by TNF-alpha, LPS, and IL-1 beta. Science. 1989 Mar 17;243(4897):1467–1469. doi: 10.1126/science.2648570. [DOI] [PubMed] [Google Scholar]
- Strieter R. M., Phan S. H., Showell H. J., Remick D. G., Lynch J. P., Genord M., Raiford C., Eskandari M., Marks R. M., Kunkel S. L. Monokine-induced neutrophil chemotactic factor gene expression in human fibroblasts. J Biol Chem. 1989 Jun 25;264(18):10621–10626. [PubMed] [Google Scholar]
- Strieter R. M., Wiggins R., Phan S. H., Wharram B. L., Showell H. J., Remick D. G., Chensue S. W., Kunkel S. L. Monocyte chemotactic protein gene expression by cytokine-treated human fibroblasts and endothelial cells. Biochem Biophys Res Commun. 1989 Jul 31;162(2):694–700. doi: 10.1016/0006-291x(89)92366-8. [DOI] [PubMed] [Google Scholar]
- Thelen M., Peveri P., Kernen P., von Tscharner V., Walz A., Baggiolini M. Mechanism of neutrophil activation by NAF, a novel monocyte-derived peptide agonist. FASEB J. 1988 Aug;2(11):2702–2706. [PubMed] [Google Scholar]
- Yoshimura T., Matsushima K., Tanaka S., Robinson E. A., Appella E., Oppenheim J. J., Leonard E. J. Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9233–9237. doi: 10.1073/pnas.84.24.9233. [DOI] [PMC free article] [PubMed] [Google Scholar]