Abstract
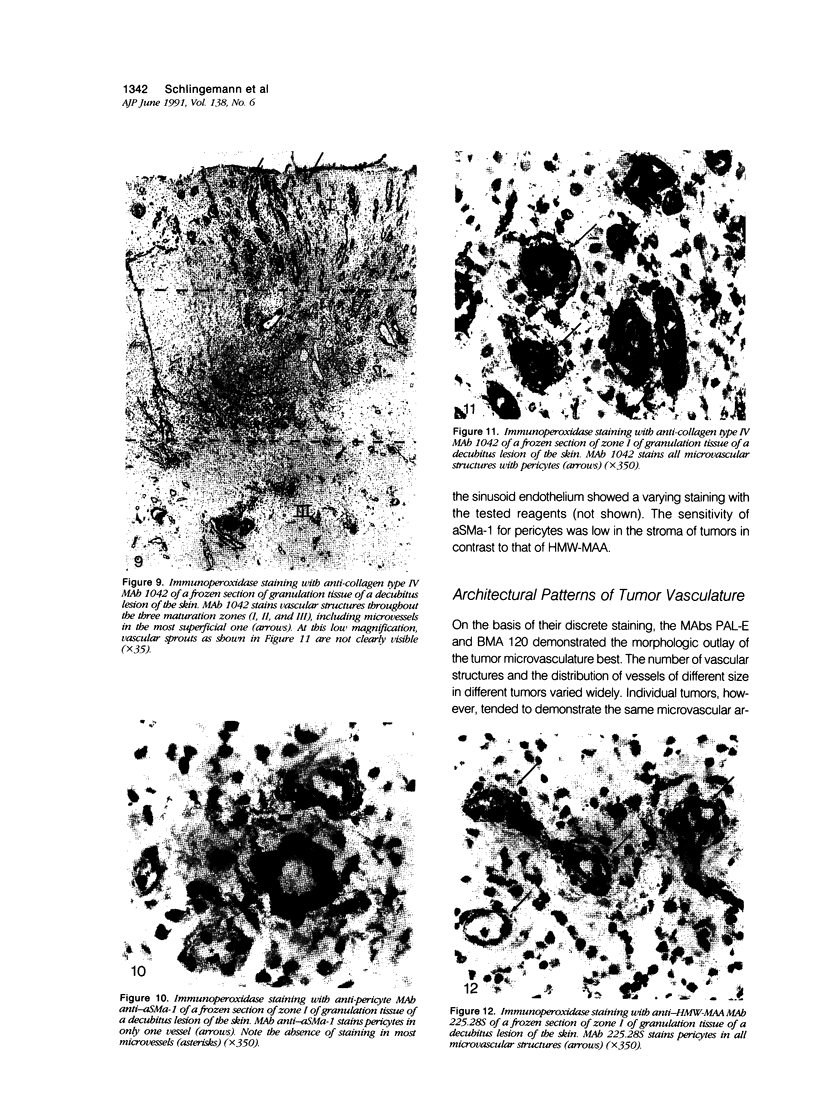
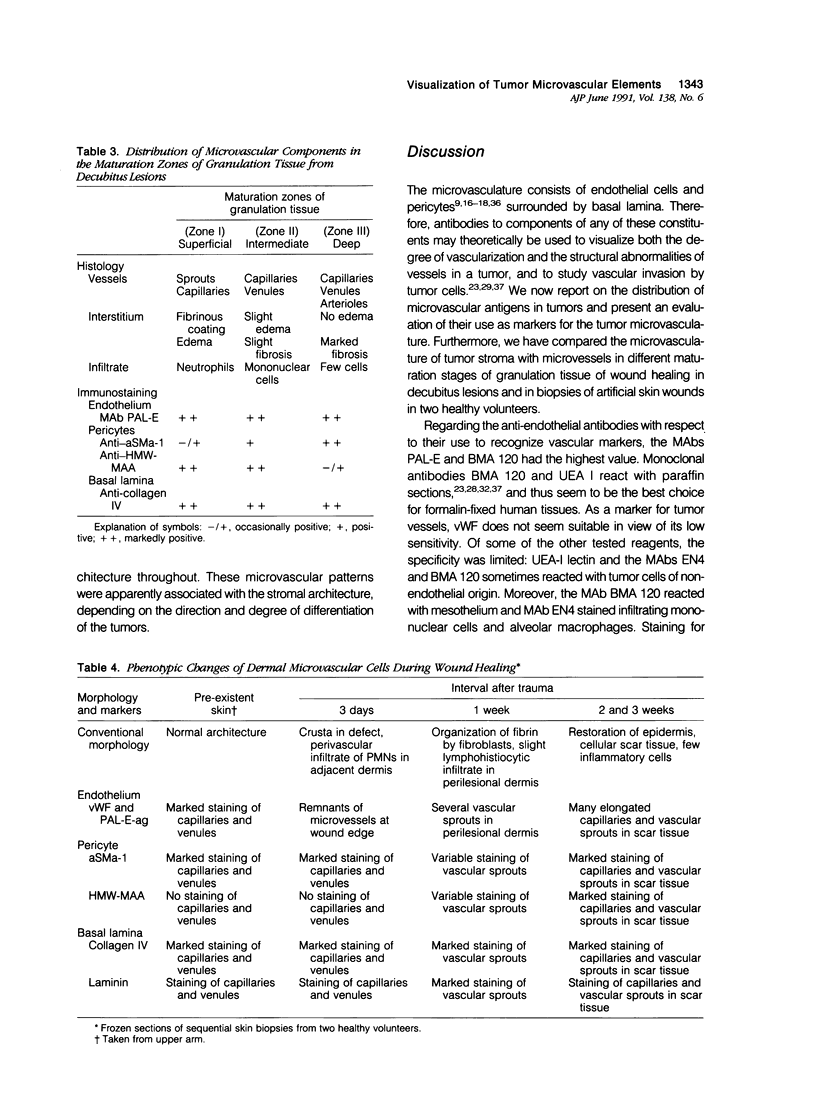
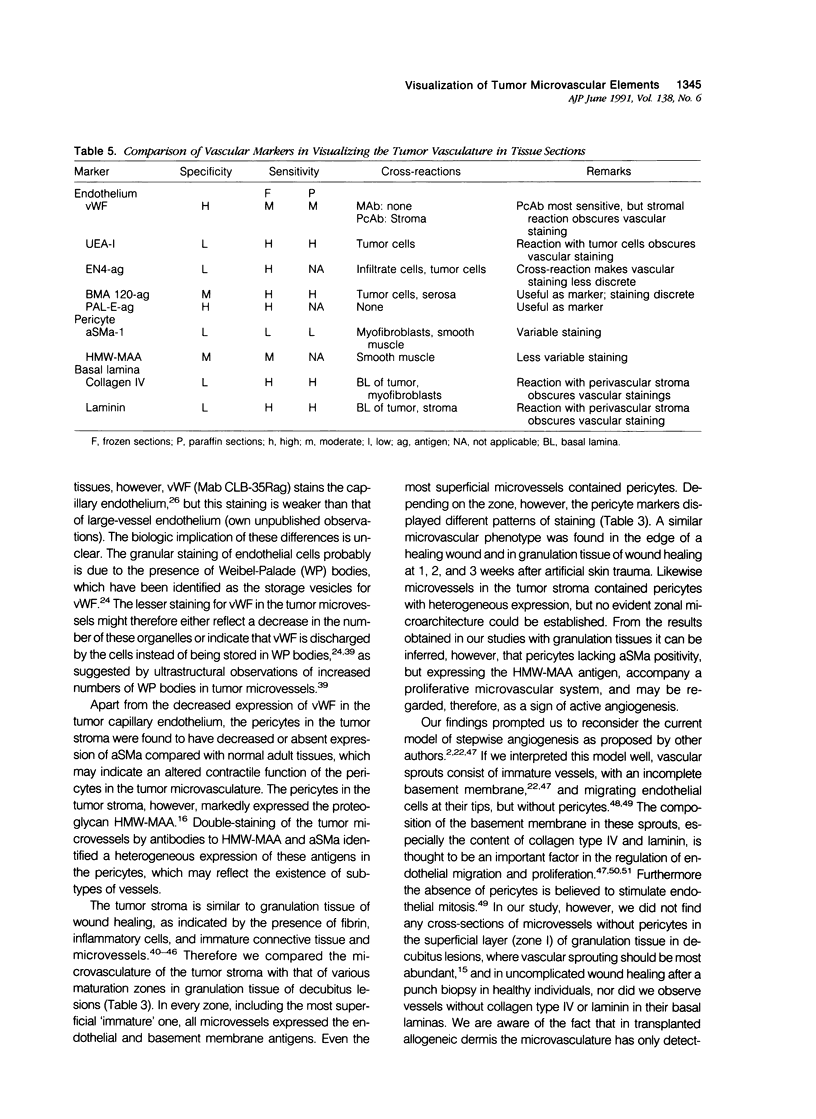
The structure and function of the tumor microvasculature is of great interest for cancer biology, diagnosis, and therapy. The distribution of endothelial cells, pericytes, and basal lamina in tumors is not well documented. In this study, the authors investigated the distribution of markers for these different components in a series of malignant human tumors and in human granulation tissue, both situations with extensive angiogenesis. Their results show a striking heterogeneity in the expression of markers for pericytes and endothelial cells between different tumors, but also within a single tumor lesion. To be able to distinguish between these two adjacent cell types decisively, all marker studies were carried out both on the light and the electron microscopical level and compared with staining results in granulation tissue of cutaneous wounds in healthy volunteers and of decubitus lesions. In granulation tissue of decubitus lesions, well-defined zones with increasing levels of maturation can be delineated. It was found that antibodies recognizing von Willebrand factor often failed to stain the tumor capillaries. Of the pericyte markers, alpha-smooth muscle actin was only locally expressed by pericytes in the tumor vasculature, whereas the high-molecular-weight melanoma-associated antigen, a chondroitin sulfate proteoglycan, stained the microvasculature broadly. Staining of the basal lamina components collagen type IV and laminin was, within the tumor, not restricted to the microvasculature. From their findings the authors conclude that 1) for the visualization of the tumor vasculature, antibodies recognizing endothelial markers, especially monoclonal antibodies PAL-E and BMA 120, are preferable to those recognizing pericytes or basal lamina; 2) within the microvasculature of tumors and granulation tissue, a heterogeneity of expression of endothelial and pericyte markers is observed; 3) during the formation of granulation tissue, all three microvascular components can be demonstrated already in the histologically earliest stage, suggesting not only an involvement of endothelial cells but also of pericytes and basal lamina in the initial steps of angiogenesis in wound healing.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alles J. U., Bosslet K. Immunohistochemical and immunochemical characterization of a new endothelial cell-specific antigen. J Histochem Cytochem. 1986 Feb;34(2):209–214. doi: 10.1177/34.2.2418100. [DOI] [PubMed] [Google Scholar]
- Ausprunk D. H., Folkman J. Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc Res. 1977 Jul;14(1):53–65. doi: 10.1016/0026-2862(77)90141-8. [DOI] [PubMed] [Google Scholar]
- Cavallo T., Sade R., Folkman J., Cotran R. S. Ultrastructural autoradiographic studies of the early vasoproliferative response in tumor angiogenesis. Am J Pathol. 1973 Mar;70(3):345–362. [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., DellaPelle P., Manseau E., Lanigan J. M., Dvorak H. F., Colvin R. B. Blood vessel fibronectin increases in conjunction with endothelial cell proliferation and capillary ingrowth during wound healing. J Invest Dermatol. 1982 Nov;79(5):269–276. doi: 10.1111/1523-1747.ep12500076. [DOI] [PubMed] [Google Scholar]
- Crocker D. J., Murad T. M., Geer J. C. Role of the pericyte in wound healing. An ultrastructural study. Exp Mol Pathol. 1970 Aug;13(1):51–65. doi: 10.1016/0014-4800(70)90084-5. [DOI] [PubMed] [Google Scholar]
- Denekamp J. Endothelial cell proliferation as a novel approach to targeting tumour therapy. Br J Cancer. 1982 Jan;45(1):136–139. doi: 10.1038/bjc.1982.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak H. F. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986 Dec 25;315(26):1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- Folkman J., Cotran R. Relation of vascular proliferation to tumor growth. Int Rev Exp Pathol. 1976;16:207–248. [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis. Adv Cancer Res. 1985;43:175–203. doi: 10.1016/s0065-230x(08)60946-x. [DOI] [PubMed] [Google Scholar]
- Form D. M., Pratt B. M., Madri J. A. Endothelial cell proliferation during angiogenesis. In vitro modulation by basement membrane components. Lab Invest. 1986 Nov;55(5):521–530. [PubMed] [Google Scholar]
- Furcht L. T. Critical factors controlling angiogenesis: cell products, cell matrix, and growth factors. Lab Invest. 1986 Nov;55(5):505–509. [PubMed] [Google Scholar]
- Grunt T. W., Lametschwandtner A., Karrer K., Staindl O. The angioarchitecture of the Lewis lung carcinoma in laboratory mice (a light microscopic and scanning electron microscopic study). Scan Electron Microsc. 1986;(Pt 2):557–573. [PubMed] [Google Scholar]
- Hagemeier H. H., Vollmer E., Goerdt S., Schulze-Osthoff K., Sorg C. A monoclonal antibody reacting with endothelial cells of budding vessels in tumors and inflammatory tissues, and non-reactive with normal adult tissues. Int J Cancer. 1986 Oct 15;38(4):481–488. doi: 10.1002/ijc.2910380405. [DOI] [PubMed] [Google Scholar]
- Harach H. R., Jasani B., Williams E. D. Factor VIII as a marker of endothelial cells in follicular carcinoma of the thyroid. J Clin Pathol. 1983 Sep;36(9):1050–1054. doi: 10.1136/jcp.36.9.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser L. S., Miller F. N. Differential macromolecular leakage from the vasculature of tumors. Cancer. 1986 Feb 1;57(3):461–464. doi: 10.1002/1097-0142(19860201)57:3<461::aid-cncr2820570310>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Ho K. L. Ultrastructure of cerebellar capillary hemangioblastoma. IV. Pericytes and their relationship to endothelial cells. Acta Neuropathol. 1985;67(3-4):254–264. doi: 10.1007/BF00687810. [DOI] [PubMed] [Google Scholar]
- Hobson B., Denekamp J. Endothelial proliferation in tumours and normal tissues: continuous labelling studies. Br J Cancer. 1984 Apr;49(4):405–413. doi: 10.1038/bjc.1984.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthöfer H., Virtanen I., Kariniemi A. L., Hormia M., Linder E., Miettinen A. Ulex europaeus I lectin as a marker for vascular endothelium in human tissues. Lab Invest. 1982 Jul;47(1):60–66. [PubMed] [Google Scholar]
- Jain R. K. Determinants of tumor blood flow: a review. Cancer Res. 1988 May 15;48(10):2641–2658. [PubMed] [Google Scholar]
- KUWABARA T., COGAN D. G. Retinal vascular patterns. VI. Mural cells of the retinal capillaries. Arch Ophthalmol. 1963 Apr;69:492–502. doi: 10.1001/archopht.1963.00960040498013. [DOI] [PubMed] [Google Scholar]
- Kumar P., Kumar S., Marsden H. B., Lynch P. G., Earnshaw E. Weibel-Palade bodies in endothelial cells as a marker for angiogenesis in brain tumors. Cancer Res. 1980 Jun;40(6):2010–2019. [PubMed] [Google Scholar]
- Langdon R. C., Cuono C. B., Birchall N., Madri J. A., Kuklinska E., McGuire J., Moellmann G. E. Reconstitution of structure and cell function in human skin grafts derived from cryopreserved allogeneic dermis and autologous cultured keratinocytes. J Invest Dermatol. 1988 Nov;91(5):478–485. doi: 10.1111/1523-1747.ep12476623. [DOI] [PubMed] [Google Scholar]
- Laurie G. W., Leblond C. P., Martin G. R. Localization of type IV collagen, laminin, heparan sulfate proteoglycan, and fibronectin to the basal lamina of basement membranes. J Cell Biol. 1982 Oct;95(1):340–344. doi: 10.1083/jcb.95.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechago J., Sun N. C., Weinstein W. M. Simultaneous visualization of two antigens in the same tissue section by combining immunoperoxidase with immunofluorescence techniques. J Histochem Cytochem. 1979 Sep;27(9):1221–1225. doi: 10.1177/27.9.113453. [DOI] [PubMed] [Google Scholar]
- McLone D. G. Ultrastructure of the vasculature of central nervous system tumors of childhood. Childs Brain. 1980;6(5):242–254. doi: 10.1159/000119910. [DOI] [PubMed] [Google Scholar]
- Murray J. C., Randhawa V., Denekamp J. The effects of melphalan and misonidazole on the vasculature of a murine sarcoma. Br J Cancer. 1987 Mar;55(3):233–238. doi: 10.1038/bjc.1987.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane P K, Pierce G B., Jr Enzyme-labeled antibodies: preparation and application for the localization of antigens. J Histochem Cytochem. 1966 Dec;14(12):929–931. doi: 10.1177/14.12.929. [DOI] [PubMed] [Google Scholar]
- Nawroth P., Handley D., Matsueda G., De Waal R., Gerlach H., Blohm D., Stern D. Tumor necrosis factor/cachectin-induced intravascular fibrin formation in meth A fibrosarcomas. J Exp Med. 1988 Aug 1;168(2):637–647. doi: 10.1084/jem.168.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlidge A., D'Amore P. A. Inhibition of capillary endothelial cell growth by pericytes and smooth muscle cells. J Cell Biol. 1987 Sep;105(3):1455–1462. doi: 10.1083/jcb.105.3.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold H. S., van den Berg-Blok A. Factors influencing the neovascularization of experimental tumours. Biorheology. 1984;21(4):493–501. doi: 10.3233/bir-1984-21408. [DOI] [PubMed] [Google Scholar]
- Rofstad E. K. Growth and vascular structure of human melanoma xenografts. Cell Tissue Kinet. 1984 Jan;17(1):91–101. doi: 10.1111/j.1365-2184.1984.tb00571.x. [DOI] [PubMed] [Google Scholar]
- Ruiter D. J., Schlingemann R. O., Rietveld F. J., de Waal R. M. Monoclonal antibody-defined human endothelial antigens as vascular markers. J Invest Dermatol. 1989 Aug;93(2 Suppl):25S–32S. doi: 10.1111/1523-1747.ep12580902. [DOI] [PubMed] [Google Scholar]
- SCHOEFL G. I. STUDIES ON INFLAMMATION. III. GROWING CAPILLARIES: THEIR STRUCTURE AND PERMEABILITY. Virchows Arch Pathol Anat Physiol Klin Med. 1963 Nov 8;337:97–141. [PubMed] [Google Scholar]
- Sawada T., Nakamura M., Sakurai I. An immunohistochemical study of neovasculature in human brain tumors. Acta Pathol Jpn. 1988 Jun;38(6):713–721. doi: 10.1111/j.1440-1827.1988.tb02343.x. [DOI] [PubMed] [Google Scholar]
- Schlingemann R. O., Dingjan G. M., Emeis J. J., Blok J., Warnaar S. O., Ruiter D. J. Monoclonal antibody PAL-E specific for endothelium. Lab Invest. 1985 Jan;52(1):71–76. [PubMed] [Google Scholar]
- Schlingemann R. O., Rietveld F. J., de Waal R. M., Bradley N. J., Skene A. I., Davies A. J., Greaves M. F., Denekamp J., Ruiter D. J. Leukocyte antigen CD34 is expressed by a subset of cultured endothelial cells and on endothelial abluminal microprocesses in the tumor stroma. Lab Invest. 1990 Jun;62(6):690–696. [PubMed] [Google Scholar]
- Schlingemann R. O., Rietveld F. J., de Waal R. M., Ferrone S., Ruiter D. J. Expression of the high molecular weight melanoma-associated antigen by pericytes during angiogenesis in tumors and in healing wounds. Am J Pathol. 1990 Jun;136(6):1393–1405. [PMC free article] [PubMed] [Google Scholar]
- Schor A. M., Schor S. L. Tumour angiogenesis. J Pathol. 1983 Nov;141(3):385–413. doi: 10.1002/path.1711410315. [DOI] [PubMed] [Google Scholar]
- Scopsi L., Larsson L. I. Increased sensitivity in peroxidase immunocytochemistry. A comparative study of a number of peroxidase visualization methods employing a model system. Histochemistry. 1986;84(3):221–230. doi: 10.1007/BF00495786. [DOI] [PubMed] [Google Scholar]
- Seemayer T. A., Lagacé R., Schürch W., Tremblay G. Myofibroblasts in the stroma of invasive and metastatic carcinoma: a possible host response to neoplasia. Am J Surg Pathol. 1979 Dec;3(6):525–533. doi: 10.1097/00000478-197912000-00005. [DOI] [PubMed] [Google Scholar]
- Sims D. E. The pericyte--a review. Tissue Cell. 1986;18(2):153–174. doi: 10.1016/0040-8166(86)90026-1. [DOI] [PubMed] [Google Scholar]
- Skalli O., Ropraz P., Trzeciak A., Benzonana G., Gillessen D., Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986 Dec;103(6 Pt 2):2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock I. F. Population kinetics of carcinoma cells, capillary endothelial cells, and fibroblasts in a transplanted mouse mammary tumor. Cancer Res. 1970 Oct;30(10):2470–2476. [PubMed] [Google Scholar]
- Wakui S. Two- and three-dimensional ultrastructural observation of two cell angiogenesis in human granulation tissue. Virchows Arch B Cell Pathol Incl Mol Pathol. 1988;56(2):127–139. doi: 10.1007/BF02890011. [DOI] [PubMed] [Google Scholar]
- Weber K., Braun-Falco O. Ultrastructure of blood vessels in human granulation tissue. Arch Dermatol Forsch. 1973;248(1):29–44. doi: 10.1007/BF00594710. [DOI] [PubMed] [Google Scholar]
- van der Kwast T. H., Stel H. V., Cristen E., Bertina R. M., Veerman E. C. Localization of factor VIII-procoagulant antigen: an immunohistological survey of the human body using monoclonal antibodies. Blood. 1986 Jan;67(1):222–227. [PubMed] [Google Scholar]