Abstract
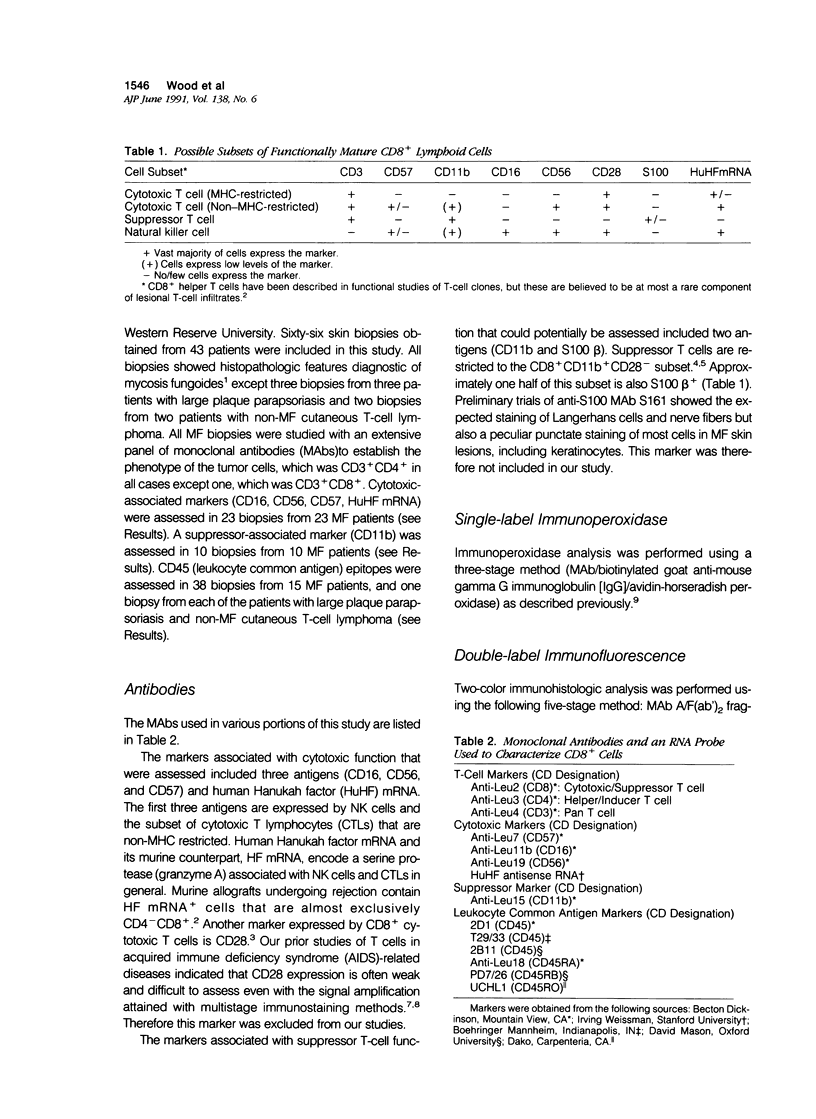
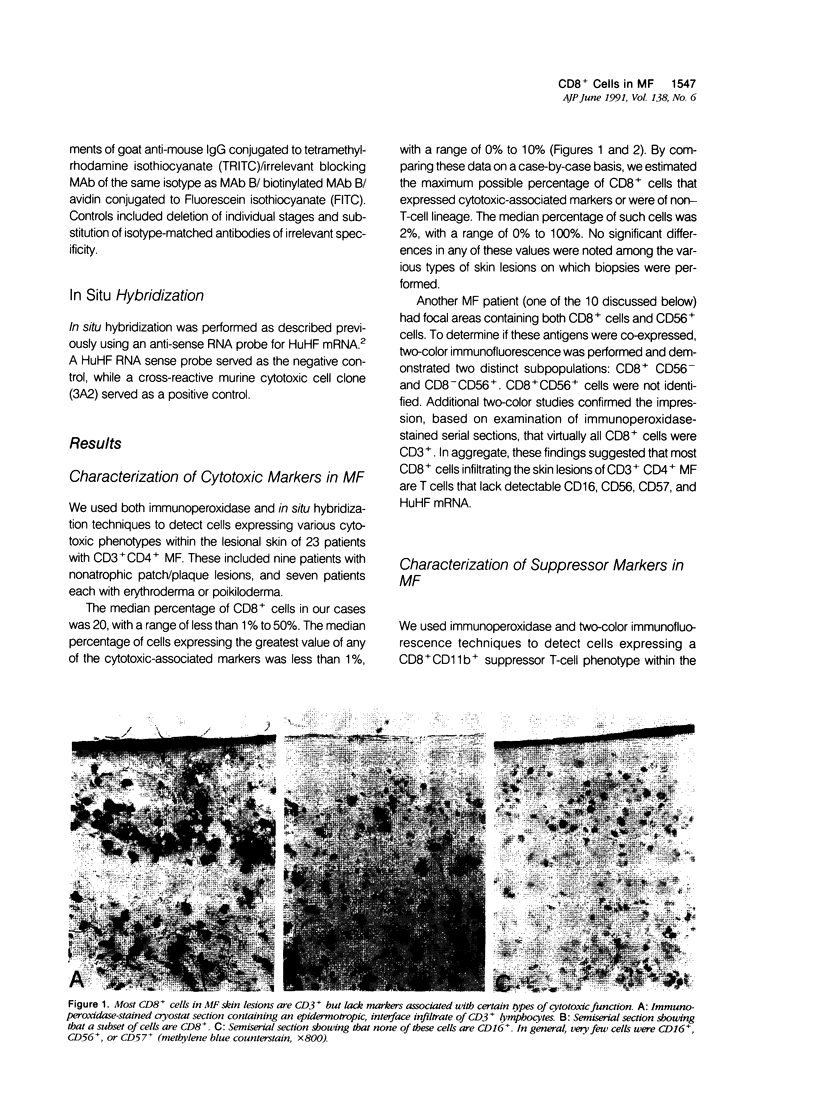
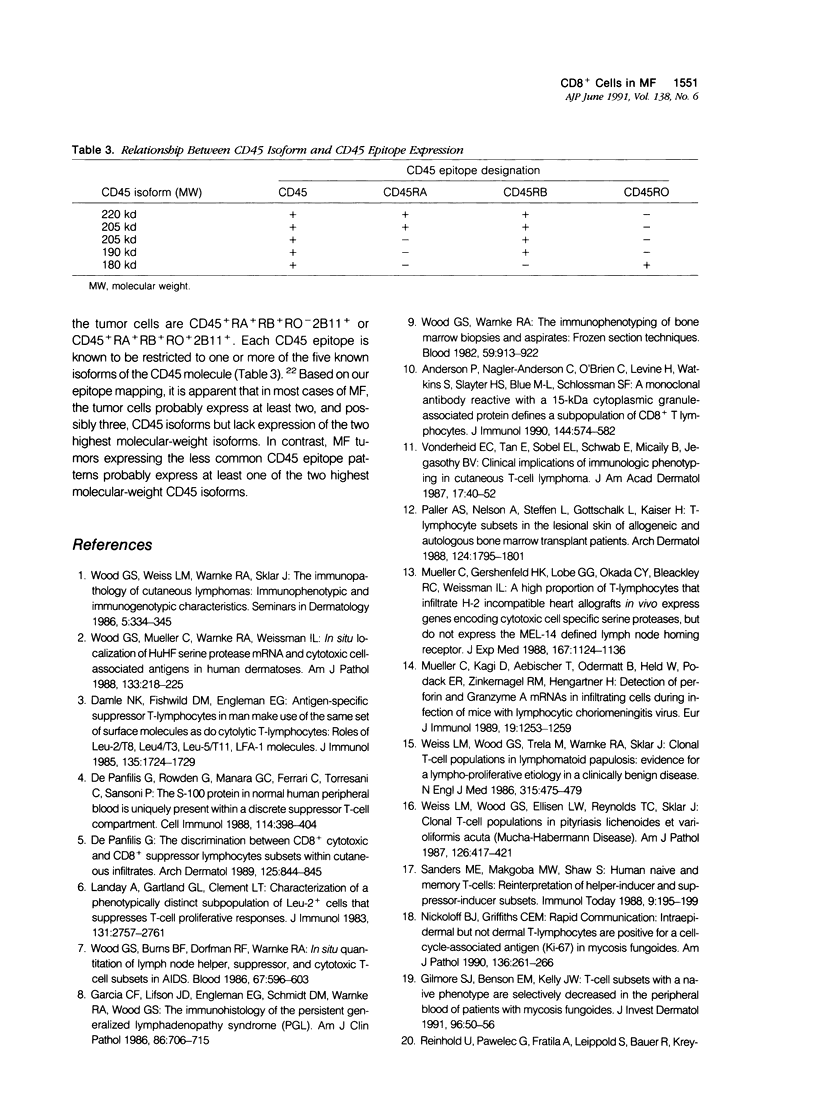
To define further the characteristics of CD8+ cells in skin lesions of CD3+ CD4+ mycosis fungoides (MF), the authors used single- and double-label immunohistologic techniques and in situ hybridization to detect antigens and transcripts associated with certain types of cytotoxic or suppressor function. The cytotoxic markers included CD16, CD56, CD57, and an anti-sense probe for human Hanukah factor (HuHf) mRNA. Analysis of 23 cases demonstrated that lesional CD8+ cells were CD3+ T cells that generally lacked expression of any of the cytotoxic markers studied. Analysis of another 10 cases confirmed the CD3+ T-cell lineage of lesional CD8+ cells and demonstrated that these cells also lacked expression of the suppressor-associated marker, CD11b. In aggregate, these results indicate that most CD8+ cells in CD3+ CD4+ MF skin lesions are of T-cell rather than NK-cell differentiation. Their overall phenotype suggests that they may be major histocompatibility complex (MHC)-restricted cytotoxic T cells lacking appreciable levels of HuHF serine protease. Because the induction of CD8+ suppressor T cells is mediated by CD4+ T cells expressing the CD45RA+ RO- phenotype, CD45 epitope expression was studied in 15 MF cases. The vast majority (13/15) contained CD3+ CD4+ tumor cells that were CD45+ RA- RB+ RO+ 2B11+. This phenotype is consistent with memory T cells rather than suppressor-inducer T cells, and correlates with the paucity of phenotypically defined suppressor T cells in CD3+ CD4+ MF skin lesions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., Nagler-Anderson C., O'Brien C., Levine H., Watkins S., Slayter H. S., Blue M. L., Schlossman S. F. A monoclonal antibody reactive with a 15-kDa cytoplasmic granule-associated protein defines a subpopulation of CD8+ T lymphocytes. J Immunol. 1990 Jan 15;144(2):574–582. [PubMed] [Google Scholar]
- Damle N. K., Fishwild D. M., Engleman E. G. Antigen-specific suppressor T lymphocytes in man make use of the same set of surface molecules as do cytolytic T lymphocytes: roles of Leu-2/T8, Leu-4/T3, Leu-5/T11, LFA-1 molecules. J Immunol. 1985 Sep;135(3):1724–1730. [PubMed] [Google Scholar]
- Damle N. K., Mohagheghpour N., Engleman E. G. Soluble antigen-primed inducer T cells activate antigen-specific suppressor T cells in the absence of antigen-pulsed accessory cells: phenotypic definition of suppressor-inducer and suppressor-effector cells. J Immunol. 1984 Feb;132(2):644–650. [PubMed] [Google Scholar]
- De Panfilis G., Rowden G., Manara G. C., Ferrari C., Torresani C., Sansoni P. The S-100 beta protein in normal human peripheral blood is uniquely present within a discrete suppressor-T-cell compartment. Cell Immunol. 1988 Jul;114(2):398–404. doi: 10.1016/0008-8749(88)90331-0. [DOI] [PubMed] [Google Scholar]
- De Panfilis G. The discrimination between CD8+ cytotoxic and CD8+ suppressor lymphocyte subsets within cutaneous infiltrates. Arch Dermatol. 1989 Jun;125(6):844–845. [PubMed] [Google Scholar]
- Garcia C. F., Lifson J. D., Engleman E. G., Schmidt D. M., Warnke R. A., Wood G. S. The immunohistology of the persistent generalized lymphadenopathy syndrome (PGL). Am J Clin Pathol. 1986 Dec;86(6):706–715. doi: 10.1093/ajcp/86.6.706. [DOI] [PubMed] [Google Scholar]
- Gilmore S. J., Benson E. M., Kelly J. W. T-cell subsets with a naive phenotype are selectively decreased in the peripheral blood of patients with mycosis fungoides. J Invest Dermatol. 1991 Jan;96(1):50–56. doi: 10.1111/1523-1747.ep12514722. [DOI] [PubMed] [Google Scholar]
- Landay A., Gartland G. L., Clement L. T. Characterization of a phenotypically distinct subpopulation of Leu-2+ cells that suppresses T cell proliferative responses. J Immunol. 1983 Dec;131(6):2757–2761. [PubMed] [Google Scholar]
- Mueller C., Gershenfeld H. K., Lobe C. G., Okada C. Y., Bleackley R. C., Weissman I. L. A high proportion of T lymphocytes that infiltrate H-2-incompatible heart allografts in vivo express genes encoding cytotoxic cell-specific serine proteases, but do not express the MEL-14-defined lymph node homing receptor. J Exp Med. 1988 Mar 1;167(3):1124–1136. doi: 10.1084/jem.167.3.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller C., Kägi D., Aebischer T., Odermatt B., Held W., Podack E. R., Zinkernagel R. M., Hengartner H. Detection of perforin and granzyme A mRNA in infiltrating cells during infection of mice with lymphocytic choriomeningitis virus. Eur J Immunol. 1989 Jul;19(7):1253–1259. doi: 10.1002/eji.1830190716. [DOI] [PubMed] [Google Scholar]
- Nickoloff B. J., Griffiths C. E. Intraepidermal but not dermal T lymphocytes are positive for a cell-cycle-associated antigen (Ki-67) in mycosis fungoides. Am J Pathol. 1990 Feb;136(2):261–266. [PMC free article] [PubMed] [Google Scholar]
- Paller A. S., Nelson A., Steffen L., Gottschalk L., Kaizer H. T-lymphocyte subsets in the lesional skin of allogeneic and autologous bone marrow transplant patients. Arch Dermatol. 1988 Dec;124(12):1795–1801. [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Shaw S. Human naive and memory T cells: reinterpretation of helper-inducer and suppressor-inducer subsets. Immunol Today. 1988 Jul-Aug;9(7-8):195–199. doi: 10.1016/0167-5699(88)91212-1. [DOI] [PubMed] [Google Scholar]
- Streuli M., Morimoto C., Schrieber M., Schlossman S. F., Saito H. Characterization of CD45 and CD45R monoclonal antibodies using transfected mouse cell lines that express individual human leukocyte common antigens. J Immunol. 1988 Dec 1;141(11):3910–3914. [PubMed] [Google Scholar]
- Vonderheid E. C., Tan E., Sobel E. L., Schwab E., Micaily B., Jegasothy B. V. Clinical implications of immunologic phenotyping in cutaneous T cell lymphoma. J Am Acad Dermatol. 1987 Jul;17(1):40–52. doi: 10.1016/s0190-9622(87)70168-6. [DOI] [PubMed] [Google Scholar]
- Weiss L. M., Wood G. S., Ellisen L. W., Reynolds T. C., Sklar J. Clonal T-cell populations in pityriasis lichenoides et varioliformis acuta (Mucha-Habermann disease). Am J Pathol. 1987 Mar;126(3):417–421. [PMC free article] [PubMed] [Google Scholar]
- Weiss L. M., Wood G. S., Trela M., Warnke R. A., Sklar J. Clonal T-cell populations in lymphomatoid papulosis. Evidence of a lymphoproliferative origin for a clinically benign disease. N Engl J Med. 1986 Aug 21;315(8):475–479. doi: 10.1056/NEJM198608213150802. [DOI] [PubMed] [Google Scholar]
- Wood G. S., Burns B. F., Dorfman R. F., Warnke R. A. In situ quantitation of lymph node helper, suppressor, and cytotoxic T cell subsets in AIDS. Blood. 1986 Mar;67(3):596–603. [PubMed] [Google Scholar]
- Wood G. S., Mueller C., Warnke R. A., Weissman I. L. In situ localization of HuHF serine protease mRNA and cytotoxic cell-associated antigens in human dermatoses. A novel method for the detection of cytotoxic cells in human tissues. Am J Pathol. 1988 Nov;133(2):218–225. [PMC free article] [PubMed] [Google Scholar]
- Wood G. S., Warnke R. A. The immunologic phenotyping of bone marrow biopsies and aspirates: frozen section techniques. Blood. 1982 May;59(5):913–922. [PubMed] [Google Scholar]






