Abstract
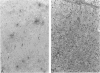
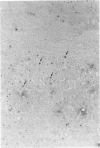
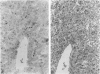
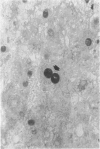
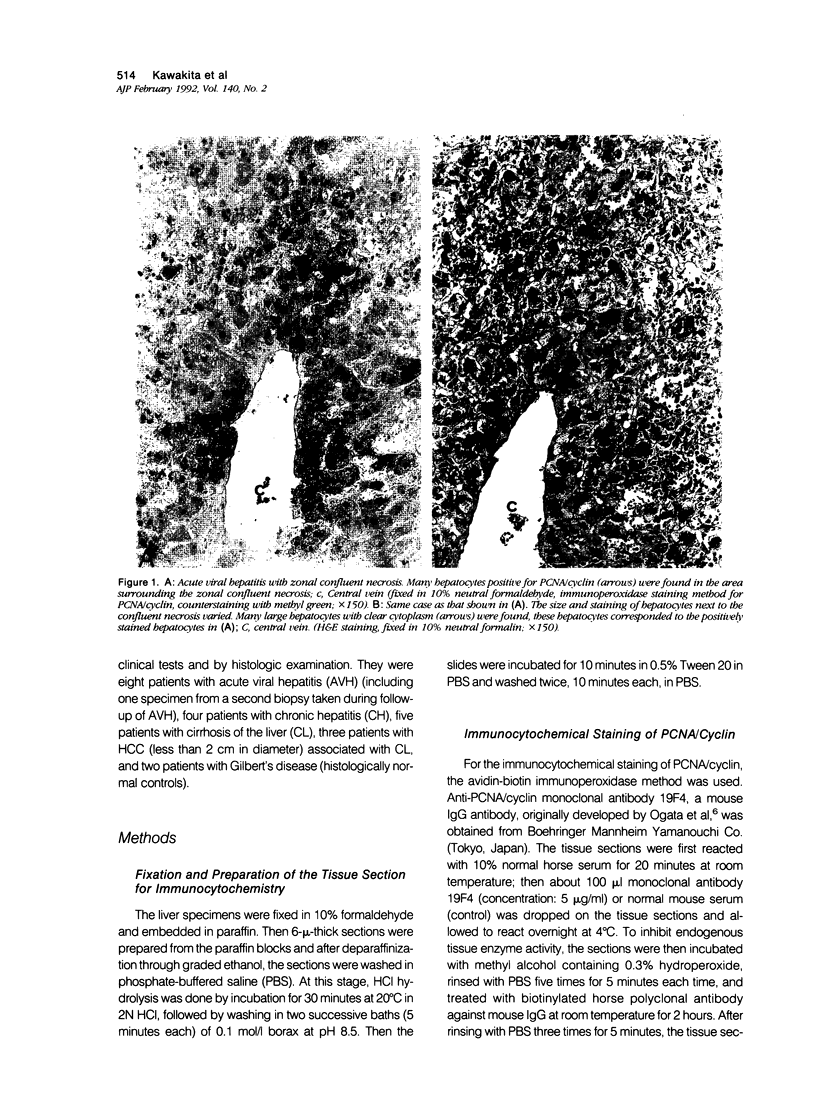
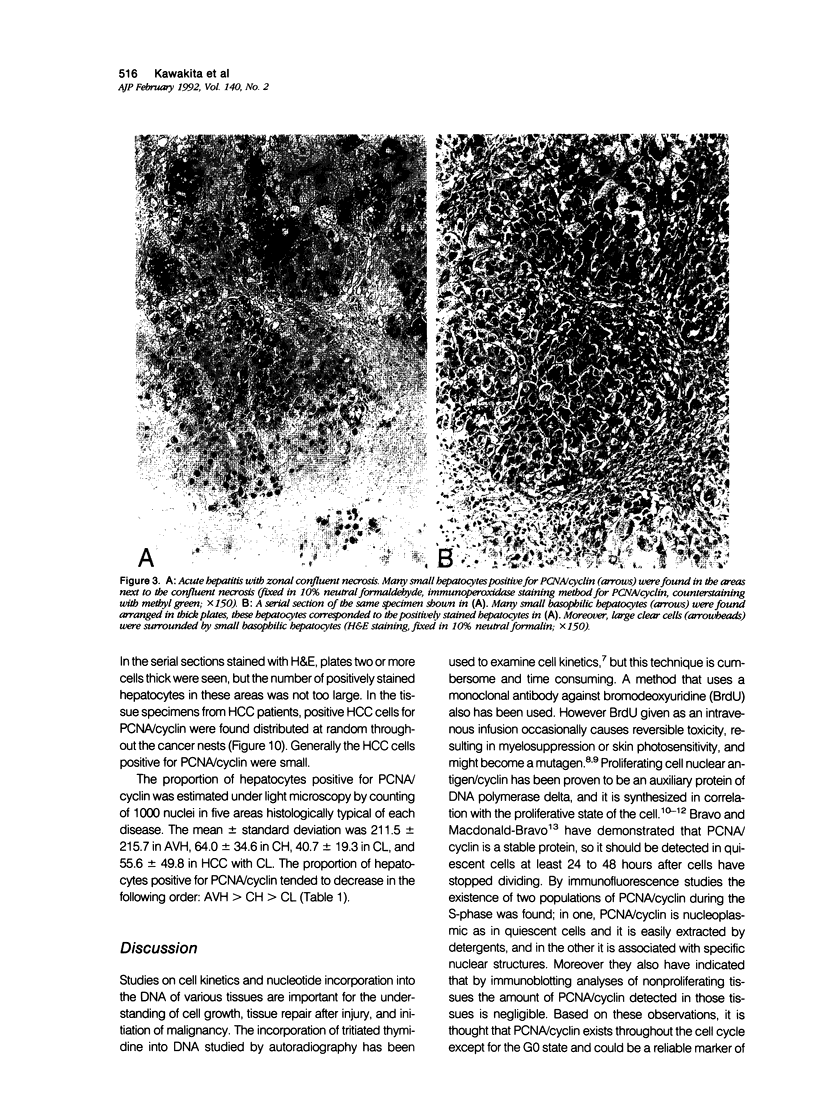
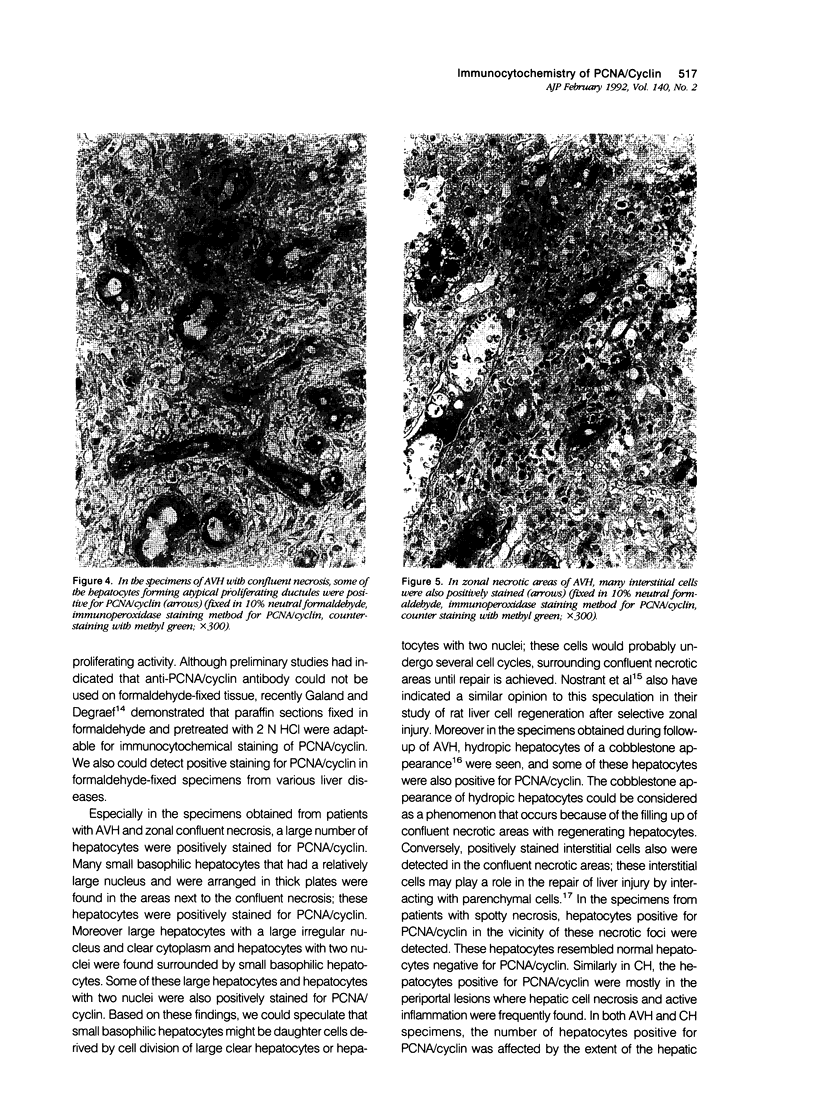
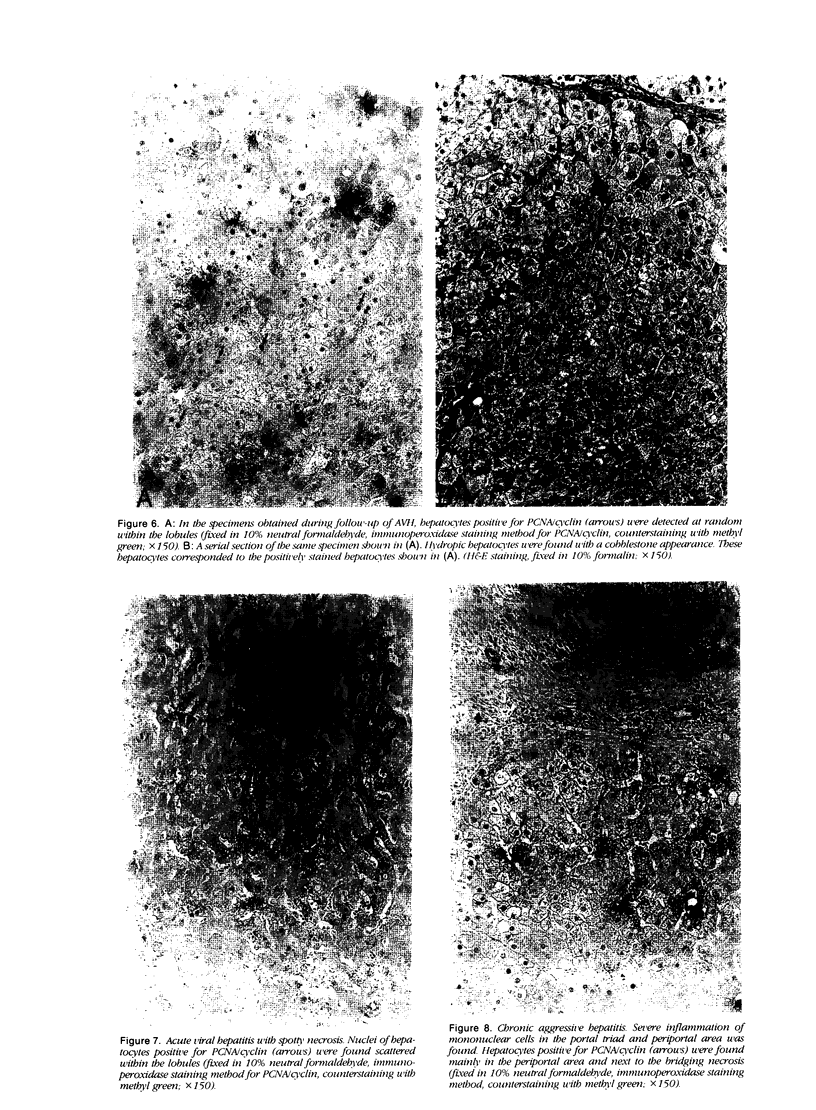
The authors studied histochemically the morphologic features of proliferating hepatocytes positive for proliferating cell nuclear antigen (PCNA/cyclin) to analyze the process of liver regeneration in embedded tissues fixed with formaldehyde using an anti-PCNA/cyclin monoclonal antibody. In liver specimens from patients with acute viral hepatitis (AVH) and confluent necrosis, many small basophilic hepatocytes surrounding large clear hepatocytes were positively stained in the areas next to the confluent necrosis. Therefore these small hepatocytes may be daughter cells derived from large clear hepatocytes that probably enter the mitotic cell cycle repeatedly to repair a large necrotic area. In the case of AVH with spotty necrosis, the positively stained hepatocytes were scattered around the necrotic foci. In the liver specimens from patients with chronic active hepatitis, most of the positively stained hepatocytes were located next to the necrotic area. As for cirrhosis of the liver, the number of hepatocytes positive for PCNA/cyclin varied greatly in different pseudolobules, and in the specimens of hepatocellular carcinoma (HCC), the HCC cells positive for PCNA/cyclin were detected throughout the cancer nests.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bravo R., Celis J. E. A search for differential polypeptide synthesis throughout the cell cycle of HeLa cells. J Cell Biol. 1980 Mar;84(3):795–802. doi: 10.1083/jcb.84.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Frank R., Blundell P. A., Macdonald-Bravo H. Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta. Nature. 1987 Apr 2;326(6112):515–517. doi: 10.1038/326515a0. [DOI] [PubMed] [Google Scholar]
- Bravo R., Macdonald-Bravo H. Changes in the nuclear distribution of cyclin (PCNA) but not its synthesis depend on DNA replication. EMBO J. 1985 Mar;4(3):655–661. doi: 10.1002/j.1460-2075.1985.tb03679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Macdonald-Bravo H. Existence of two populations of cyclin/proliferating cell nuclear antigen during the cell cycle: association with DNA replication sites. J Cell Biol. 1987 Oct;105(4):1549–1554. doi: 10.1083/jcb.105.4.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celis J. E., Celis A. Cell cycle-dependent variations in the distribution of the nuclear protein cyclin proliferating cell nuclear antigen in cultured cells: subdivision of S phase. Proc Natl Acad Sci U S A. 1985 May;82(10):3262–3266. doi: 10.1073/pnas.82.10.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galand P., Degraef C. Cyclin/PCNA immunostaining as an alternative to tritiated thymidine pulse labelling for marking S phase cells in paraffin sections from animal and human tissues. Cell Tissue Kinet. 1989 Sep;22(5):383–392. doi: 10.1111/j.1365-2184.1989.tb00223.x. [DOI] [PubMed] [Google Scholar]
- Garcia R. L., Coltrera M. D., Gown A. M. Analysis of proliferative grade using anti-PCNA/cyclin monoclonal antibodies in fixed, embedded tissues. Comparison with flow cytometric analysis. Am J Pathol. 1989 Apr;134(4):733–739. [PMC free article] [PubMed] [Google Scholar]
- Gratzner H. G. Monoclonal antibody to 5-bromo- and 5-iododeoxyuridine: A new reagent for detection of DNA replication. Science. 1982 Oct 29;218(4571):474–475. doi: 10.1126/science.7123245. [DOI] [PubMed] [Google Scholar]
- Jaskulski D., deRiel J. K., Mercer W. E., Calabretta B., Baserga R. Inhibition of cellular proliferation by antisense oligodeoxynucleotides to PCNA cyclin. Science. 1988 Jun 10;240(4858):1544–1546. doi: 10.1126/science.2897717. [DOI] [PubMed] [Google Scholar]
- Karvountzis G. G., Redeker A. G., Peters R. L. Long term follow-up studies of patients surviving fluminant viral hepatitis. Gastroenterology. 1974 Nov;67(5):870–877. [PubMed] [Google Scholar]
- Kenmochi K., Sugihara S., Kojiro M. Relationship of histologic grade of hepatocellular carcinoma (HCC) to tumor size, and demonstration of tumor cells of multiple different grades in single small HCC. Liver. 1987 Feb;7(1):18–26. doi: 10.1111/j.1600-0676.1987.tb00310.x. [DOI] [PubMed] [Google Scholar]
- Kurki P., Vanderlaan M., Dolbeare F., Gray J., Tan E. M. Expression of proliferating cell nuclear antigen (PCNA)/cyclin during the cell cycle. Exp Cell Res. 1986 Sep;166(1):209–219. doi: 10.1016/0014-4827(86)90520-3. [DOI] [PubMed] [Google Scholar]
- Miyachi K., Fritzler M. J., Tan E. M. Autoantibody to a nuclear antigen in proliferating cells. J Immunol. 1978 Dec;121(6):2228–2234. [PubMed] [Google Scholar]
- Morstyn G., Hsu S. M., Kinsella T., Gratzner H., Russo A., Mitchell J. B. Bromodeoxyuridine in tumors and chromosomes detected with a monoclonal antibody. J Clin Invest. 1983 Nov;72(5):1844–1850. doi: 10.1172/JCI111145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noji S., Tashiro K., Koyama E., Nohno T., Ohyama K., Taniguchi S., Nakamura T. Expression of hepatocyte growth factor gene in endothelial and Kupffer cells of damaged rat livers, as revealed by in situ hybridization. Biochem Biophys Res Commun. 1990 Nov 30;173(1):42–47. doi: 10.1016/s0006-291x(05)81018-6. [DOI] [PubMed] [Google Scholar]
- Nostrant T. T., Miller D. L., Appelman H. D., Gumucio J. J. Acinar distribution of liver cell regeneration after selective zonal injury in the rat. Gastroenterology. 1978 Aug;75(2):181–186. [PubMed] [Google Scholar]
- Ogata K., Kurki P., Celis J. E., Nakamura R. M., Tan E. M. Monoclonal antibodies to a nuclear protein (PCNA/cyclin) associated with DNA replication. Exp Cell Res. 1987 Feb;168(2):475–486. doi: 10.1016/0014-4827(87)90020-6. [DOI] [PubMed] [Google Scholar]
- Shirakawa S., Luce J. K., Tannock I., Frei E., 3rd Cell proliferation in human melanoma. J Clin Invest. 1970 Jun;49(6):1188–1199. doi: 10.1172/JCI106333. [DOI] [PMC free article] [PubMed] [Google Scholar]