Abstract
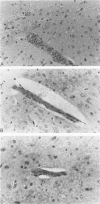
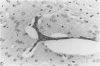
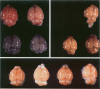
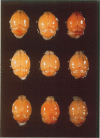
Because Plasmodium berghei ANKA induces cerebral malaria and P. vinckei does not, the former has often been studied as a model for human falciparum malaria. It lacks, however, many of the systemic changes seen in the human disease. Because both of these murine models and the human disease have now been defined in terms of excess tumor necrosis factor (TNF) production, the authors have more closely examined the two murine models in this light to see which provides the better overall model for falciparum malaria. Administering TNF to malaria-infected mice did not cause cerebral symptoms nor breakdown of the blood-brain barrier, which is the hallmark of P. berghei ANKA cerebral malaria and is generally absent in human cerebral malaria. Tumor necrosis factor did, however, induce hypoglycemia and liver injury, pathology that is seen in terminal P. vinckei and falciparum malaria, but is absent in terminal P. berghei ANKA malaria. Plasma TNF and interleukin-6 (IL-6) also were found to be consistently higher in infections caused by P. vinckei than in those caused by P. berghei ANKA. The pathology of P. vinckei malaria is thus consistent with raised systemic levels of TNF and other cytokines, as is falciparum malaria. The authors therefore conclude that P. vinckei malaria, although lacking a cerebral component, is the better model for the human disease.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarden L. A., De Groot E. R., Schaap O. L., Lansdorp P. M. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987 Oct;17(10):1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- Brouckaert P., Spriggs D. R., Demetri G., Kufe D. W., Fiers W. Circulating interleukin 6 during a continuous infusion of tumor necrosis factor and interferon gamma. J Exp Med. 1989 Jun 1;169(6):2257–2262. doi: 10.1084/jem.169.6.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher G. A., Garland T., Ajdukiewicz A. B., Clark I. A. Serum tumor necrosis factor associated with malaria in patients in the Solomon Islands. Trans R Soc Trop Med Hyg. 1990 Sep-Oct;84(5):658–661. doi: 10.1016/0035-9203(90)90134-z. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Chaudhri G. Tumour necrosis factor may contribute to the anaemia of malaria by causing dyserythropoiesis and erythrophagocytosis. Br J Haematol. 1988 Sep;70(1):99–103. doi: 10.1111/j.1365-2141.1988.tb02440.x. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Clouston W. M. Effects of endotoxin on the histology of intact and athymic mice infected with Plasmodium vinckei petteri. J Pathol. 1980 Jul;131(3):221–233. doi: 10.1002/path.1711310304. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Cowden W. B., Butcher G. A., Hunt N. H. Possible roles of tumor necrosis factor in the pathology of malaria. Am J Pathol. 1987 Oct;129(1):192–199. [PMC free article] [PubMed] [Google Scholar]
- Clark I. A. Does endotoxin cause both the disease and parasite death in acute malaria and babesiosis? Lancet. 1978 Jul 8;2(8080):75–77. doi: 10.1016/s0140-6736(78)91386-7. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Ilschner S., MacMicking J. D., Cowden W. B. TNF and Plasmodium berghei ANKA-induced cerebral malaria. Immunol Lett. 1990 Aug;25(1-3):195–198. doi: 10.1016/0165-2478(90)90114-6. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Virelizier J. L., Carswell E. A., Wood P. R. Possible importance of macrophage-derived mediators in acute malaria. Infect Immun. 1981 Jun;32(3):1058–1066. doi: 10.1128/iai.32.3.1058-1066.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau G. E., Fajardo L. F., Piguet P. F., Allet B., Lambert P. H., Vassalli P. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science. 1987 Sep 4;237(4819):1210–1212. doi: 10.1126/science.3306918. [DOI] [PubMed] [Google Scholar]
- Grau G. E., Frei K., Piguet P. F., Fontana A., Heremans H., Billiau A., Vassalli P., Lambert P. H. Interleukin 6 production in experimental cerebral malaria: modulation by anticytokine antibodies and possible role in hypergammaglobulinemia. J Exp Med. 1990 Nov 1;172(5):1505–1508. doi: 10.1084/jem.172.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau G. E., Piguet P. F., Vassalli P., Lambert P. H. Tumor-necrosis factor and other cytokines in cerebral malaria: experimental and clinical data. Immunol Rev. 1989 Dec;112:49–70. doi: 10.1111/j.1600-065x.1989.tb00552.x. [DOI] [PubMed] [Google Scholar]
- Grau G. E., Taylor T. E., Molyneux M. E., Wirima J. J., Vassalli P., Hommel M., Lambert P. H. Tumor necrosis factor and disease severity in children with falciparum malaria. N Engl J Med. 1989 Jun 15;320(24):1586–1591. doi: 10.1056/NEJM198906153202404. [DOI] [PubMed] [Google Scholar]
- Hack C. E., De Groot E. R., Felt-Bersma R. J., Nuijens J. H., Strack Van Schijndel R. J., Eerenberg-Belmer A. J., Thijs L. G., Aarden L. A. Increased plasma levels of interleukin-6 in sepsis. Blood. 1989 Oct;74(5):1704–1710. [PubMed] [Google Scholar]
- Jablons D. M., Mulé J. J., McIntosh J. K., Sehgal P. B., May L. T., Huang C. M., Rosenberg S. A., Lotze M. T. IL-6/IFN-beta-2 as a circulating hormone. Induction by cytokine administration in humans. J Immunol. 1989 Mar 1;142(5):1542–1547. [PubMed] [Google Scholar]
- Kern P., Hemmer C. J., Van Damme J., Gruss H. J., Dietrich M. Elevated tumor necrosis factor alpha and interleukin-6 serum levels as markers for complicated Plasmodium falciparum malaria. Am J Med. 1989 Aug;87(2):139–143. doi: 10.1016/s0002-9343(89)80688-6. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski D., Hill A. V., Sambou I., Twumasi P., Castracane J., Manogue K. R., Cerami A., Brewster D. R., Greenwood B. M. TNF concentration in fatal cerebral, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet. 1990 Nov 17;336(8725):1201–1204. doi: 10.1016/0140-6736(90)92827-5. [DOI] [PubMed] [Google Scholar]
- Looareesuwan S., Warrell D. A., White N. J., Sutharasamai P., Chanthavanich P., Sundaravej K., Juel-Jensen B. E., Bunnag D., Harinasuta T. Do patients with cerebral malaria have cerebral oedema? A computed tomography study. Lancet. 1983 Feb 26;1(8322):434–437. doi: 10.1016/s0140-6736(83)91437-x. [DOI] [PubMed] [Google Scholar]
- Mackey L. J., Hochmann A., June C. H., Contreras C. E., Lambert P. H. Immunopathological aspects of Plasmodium berghei infection in five strains of mice. II. Immunopathology of cerebral and other tissue lesions during the infection. Clin Exp Immunol. 1980 Dec;42(3):412–420. [PMC free article] [PubMed] [Google Scholar]
- Molyneux M. E. Cerebral malaria in children: clinical implications of cytoadherence. Am J Trop Med Hyg. 1990 Aug;43(2 Pt 2):38–41. doi: 10.4269/ajtmh.1990.43.38. [DOI] [PubMed] [Google Scholar]
- Patwari A., Aneja S., Berry A. M., Ghosh S. Hepatic dysfunction in childhood malaria. Arch Dis Child. 1979 Feb;54(2):139–141. doi: 10.1136/adc.54.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rest J. R. Cerebral malaria in inbred mice. I. A new model and its pathology. Trans R Soc Trop Med Hyg. 1982;76(3):410–415. doi: 10.1016/0035-9203(82)90203-6. [DOI] [PubMed] [Google Scholar]
- Rest J. R. Pathogenesis of cerebral malaria in golden hamsters and inbred mice. Contrib Microbiol Immunol. 1983;7:139–146. [PubMed] [Google Scholar]
- Shalaby M. R., Waage A., Aarden L., Espevik T. Endotoxin, tumor necrosis factor-alpha and interleukin 1 induce interleukin 6 production in vivo. Clin Immunol Immunopathol. 1989 Dec;53(3):488–498. doi: 10.1016/0090-1229(89)90010-x. [DOI] [PubMed] [Google Scholar]
- Sheehan K. C., Ruddle N. H., Schreiber R. D. Generation and characterization of hamster monoclonal antibodies that neutralize murine tumor necrosis factors. J Immunol. 1989 Jun 1;142(11):3884–3893. [PubMed] [Google Scholar]
- Starnes H. F., Jr, Pearce M. K., Tewari A., Yim J. H., Zou J. C., Abrams J. S. Anti-IL-6 monoclonal antibodies protect against lethal Escherichia coli infection and lethal tumor necrosis factor-alpha challenge in mice. J Immunol. 1990 Dec 15;145(12):4185–4191. [PubMed] [Google Scholar]
- Taylor T. E., Molyneux M. E., Wirima J. J., Fletcher K. A., Morris K. Blood glucose levels in Malawian children before and during the administration of intravenous quinine for severe falciparum malaria. N Engl J Med. 1988 Oct 20;319(16):1040–1047. doi: 10.1056/NEJM198810203191602. [DOI] [PubMed] [Google Scholar]
- Thumwood C. M., Hunt N. H., Clark I. A., Cowden W. B. Breakdown of the blood-brain barrier in murine cerebral malaria. Parasitology. 1988 Jun;96(Pt 3):579–589. doi: 10.1017/s0031182000080203. [DOI] [PubMed] [Google Scholar]