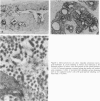
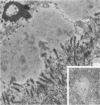
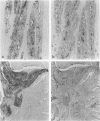
Abstract
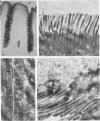
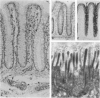
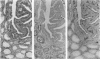
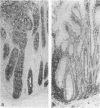
Increased sialylation of cell surface glycoconjugates has been demonstrated in malignant tumors and shown to be correlated with the invasive and metastatic growth of colon carcinoma cells. The authors have applied the Maackia amurensis lectin, which interacts with alpha 2,3-linked sialic acid, and the Sambucus nigra I lectin specific for alpha 2,6-linked sialic acid. In human colon, alpha 2,3-linked sialic acid was detectable in normal and transitional mucosa as well as in adenomas with different degrees of dysplasia and in carcinoma. In contrast, alpha 2,6-linked sialic acid as visualized with Sambucus nigra I lectin was found only in severe dysplasia and carcinoma. Thus expression of binding sites for Sambucus nigra I lectin was associated with the occurrence of histologic features of malignancy. It is concluded that malignant transformation in human colonic epithelium is accompanied by the de novo expression of an alpha 2,6 sialyl-transferase. These findings provide the basis for more detailed studies of the possible role of cell surface glycoconjugates bearing alpha 2,6-linked sialic acid in growth behavior of human colonic epithelial cells.
Full text
PDF


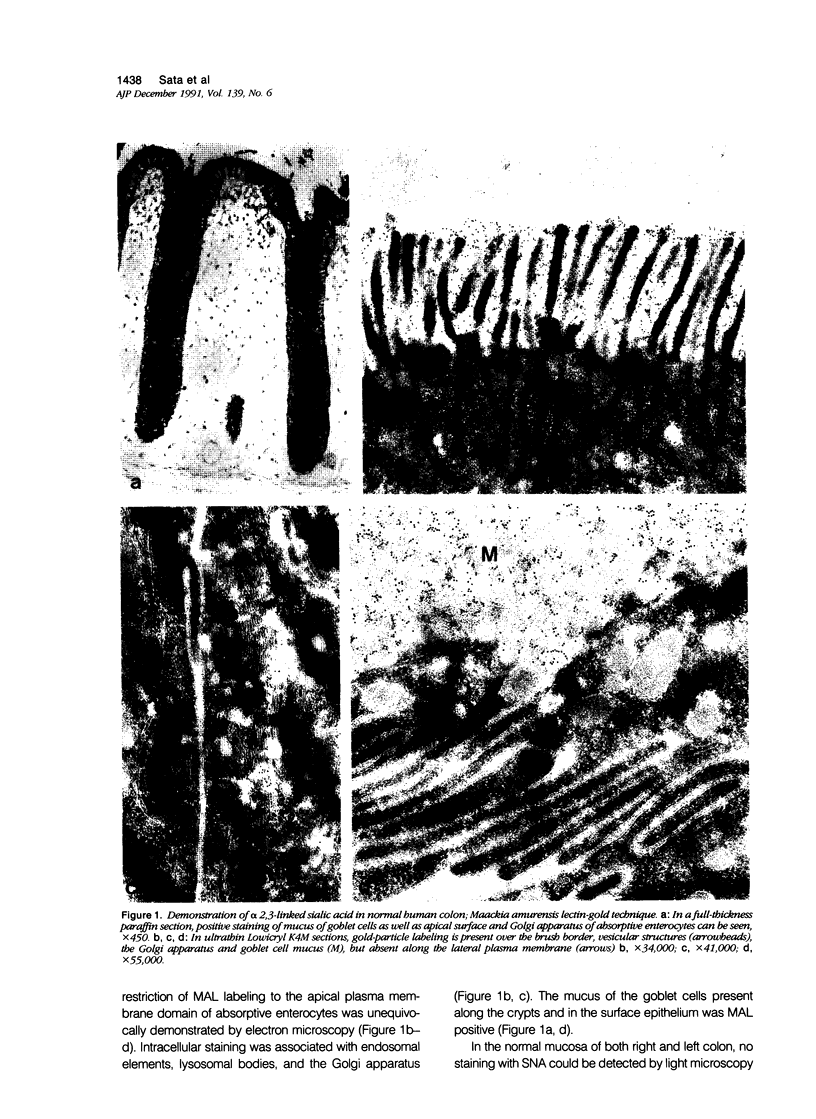
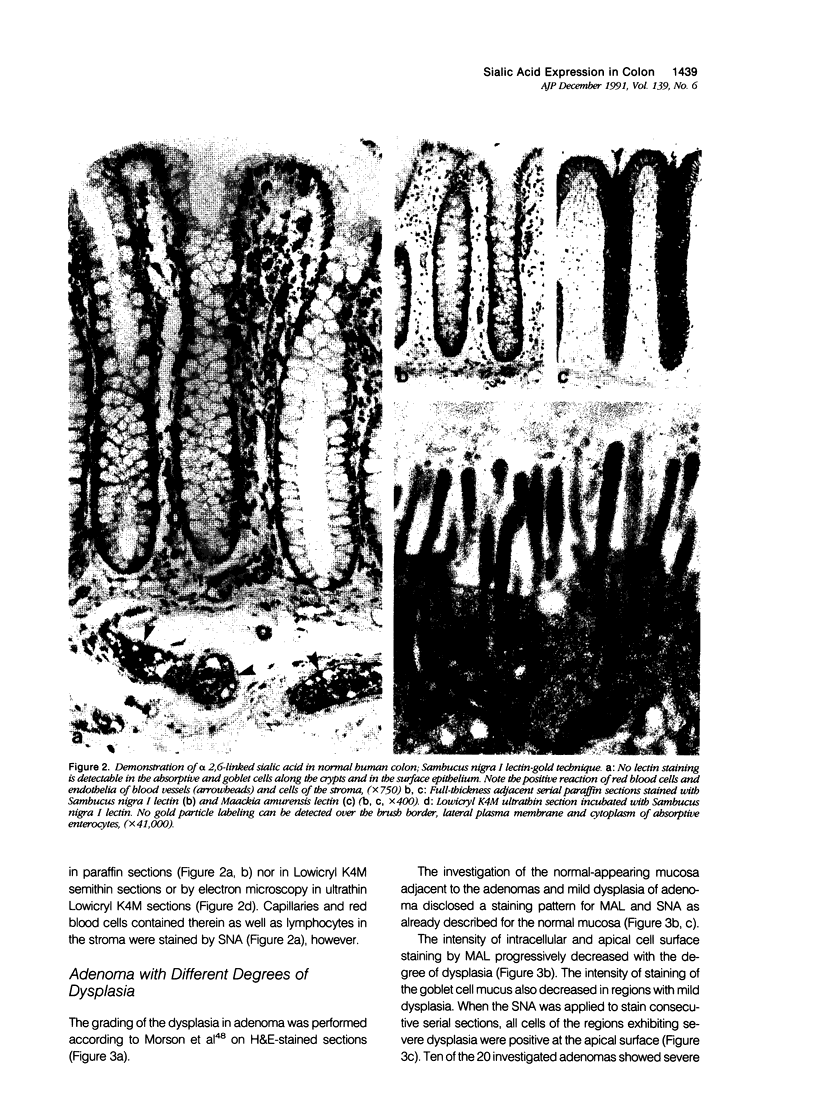
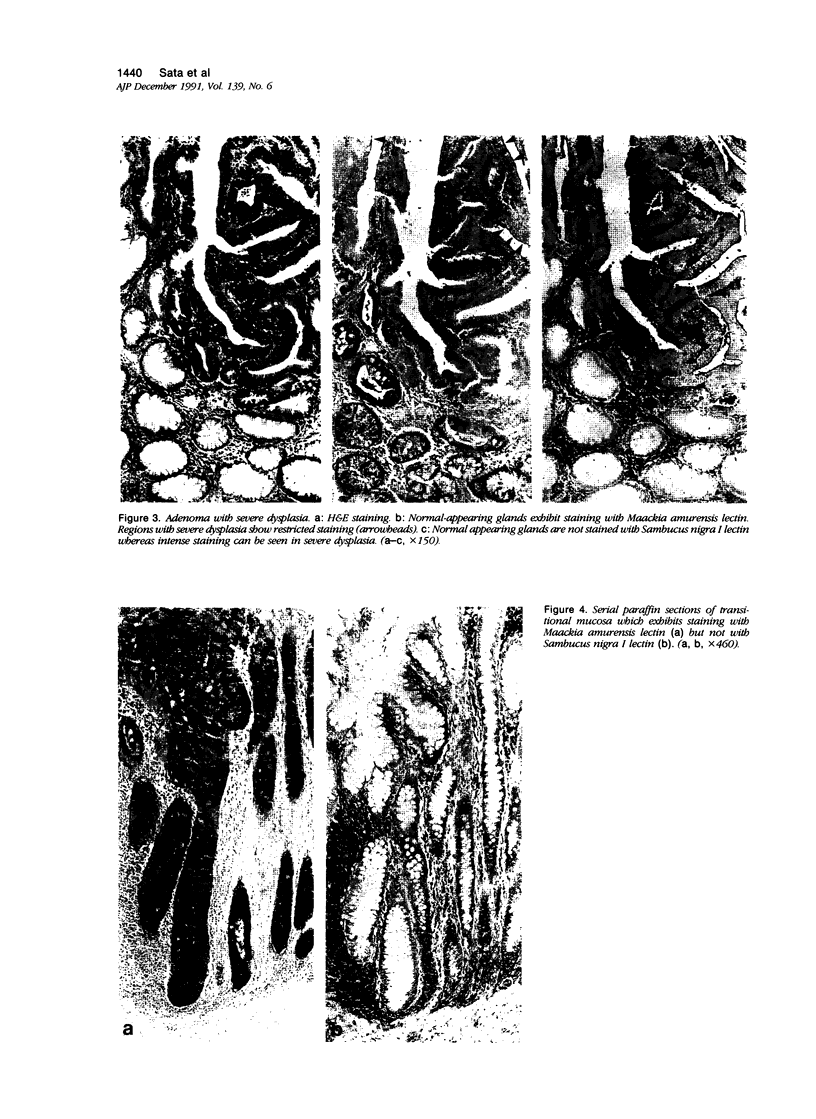
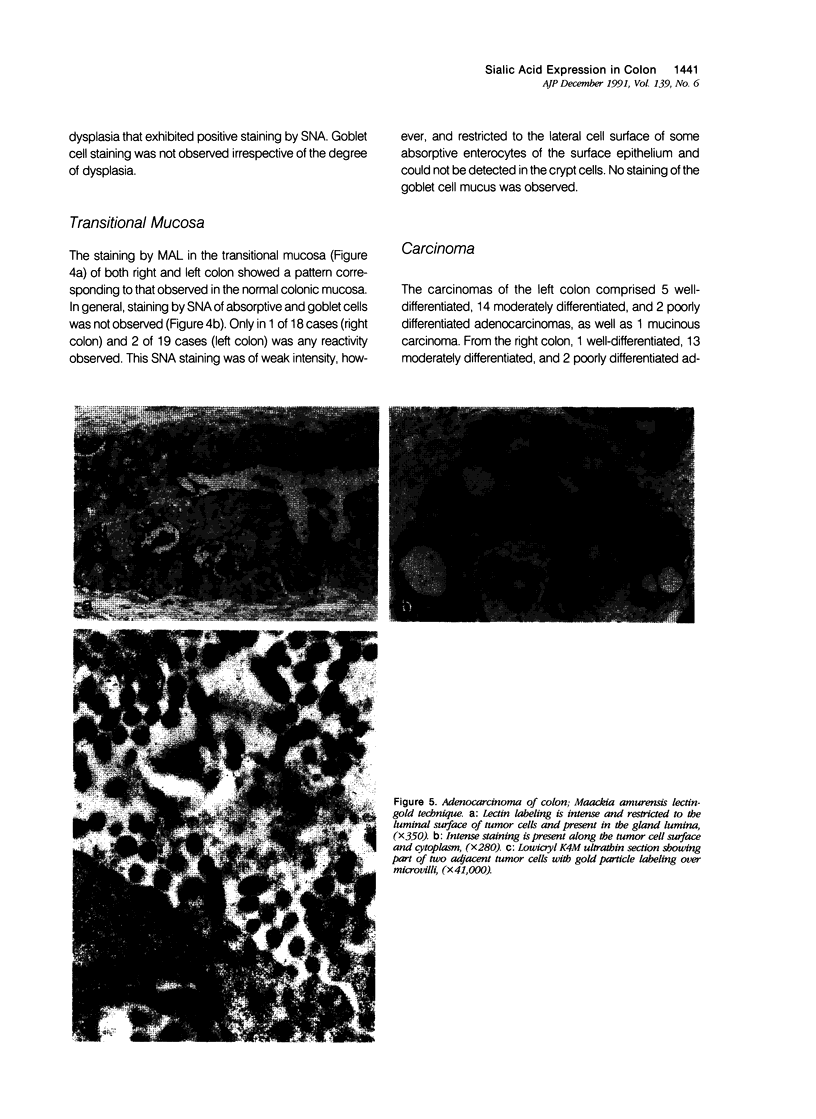

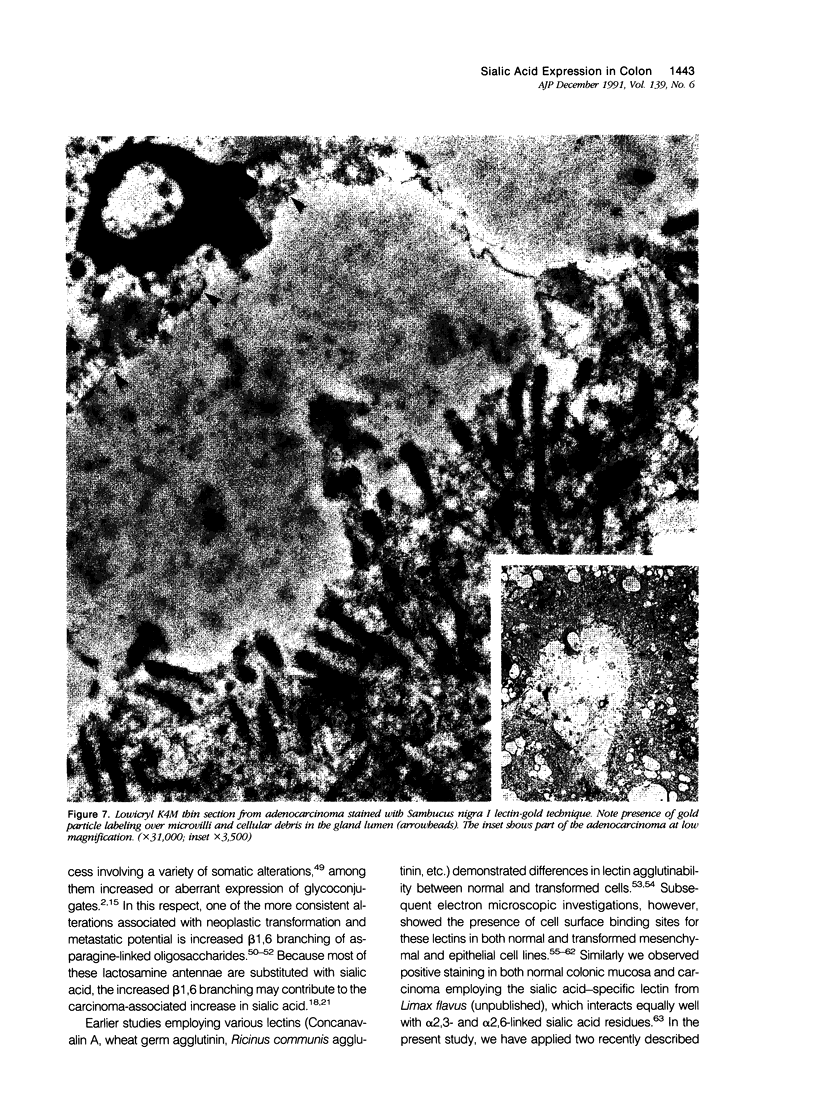




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bretton R., Wicker R., Bernhard W. Ultrastructural localization of concanavalin A receptors in normal and SV 40 -transformed hamster and rat cells. Int J Cancer. 1972 Sep 15;10(2):397–410. doi: 10.1002/ijc.2910100222. [DOI] [PubMed] [Google Scholar]
- Dennis J. W., Laferté S., Waghorne C., Breitman M. L., Kerbel R. S. Beta 1-6 branching of Asn-linked oligosaccharides is directly associated with metastasis. Science. 1987 May 1;236(4801):582–585. doi: 10.1126/science.2953071. [DOI] [PubMed] [Google Scholar]
- Ernst L. K., Rajan V. P., Larsen R. D., Ruff M. M., Lowe J. B. Stable expression of blood group H determinants and GDP-L-fucose: beta-D-galactoside 2-alpha-L-fucosyltransferase in mouse cells after transfection with human DNA. J Biol Chem. 1989 Feb 25;264(6):3436–3447. [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Fogel M., Altevogt P., Schirrmacher V. Metastatic potential severely altered by changes in tumor cell adhesiveness and cell-surface sialylation. J Exp Med. 1983 Jan 1;157(1):371–376. doi: 10.1084/jem.157.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M. N., Bothner B., Lloyd K. O., Rettig W. J., Tiller P. R., Dell A. Structures of glycosphingolipids isolated from human embryonal carcinoma cells. The presence of mono- and disialosyl glycolipids with blood group type 1 sequence. J Biol Chem. 1986 Apr 15;261(11):5145–5153. [PubMed] [Google Scholar]
- Garin-Chesa P., Rettig W. J. Immunohistochemical analysis of LNT, NeuAc2----3LNT, and Lex carbohydrate antigens in human tumors and normal tissues. Am J Pathol. 1989 Jun;134(6):1315–1327. [PMC free article] [PubMed] [Google Scholar]
- Garrido J., Burglen M. J., Samolyk D., Wicker R., Bernhard W. Ultrastructural comparison between the distribution of concanavalin A and wheat germ agglutinin cell surface receptors of normal and transformed hamster and rat cell lines. Cancer Res. 1974 Jan;34(1):230–243. [PubMed] [Google Scholar]
- Gong E., Hirohashi S., Shimosato Y., Watanabe M., Ino Y., Teshima S., Kodaira S. Expression of carbohydrate antigen 19-9 and stage-specific embryonic antigen 1 in nontumorous and tumorous epithelia of the human colon and rectum. J Natl Cancer Inst. 1985 Sep;75(3):447–454. [PubMed] [Google Scholar]
- Hakomori S. Aberrant glycosylation in cancer cell membranes as focused on glycolipids: overview and perspectives. Cancer Res. 1985 Jun;45(6):2405–2414. [PubMed] [Google Scholar]
- Hoff S. D., Matsushita Y., Ota D. M., Cleary K. R., Yamori T., Hakomori S., Irimura T. Increased expression of sialyl-dimeric LeX antigen in liver metastases of human colorectal carcinoma. Cancer Res. 1989 Dec 15;49(24 Pt 1):6883–6888. [PubMed] [Google Scholar]
- Huet C., Bernhard W. Differences in the surface mobility between normal and SV40-, polyoma- and adenovirus-transformed hamster cells. Int J Cancer. 1974 Feb 15;13(2):227–239. doi: 10.1002/ijc.2910130210. [DOI] [PubMed] [Google Scholar]
- Itzkowitz S. H., Yuan M., Montgomery C. K., Kjeldsen T., Takahashi H. K., Bigbee W. L., Kim Y. S. Expression of Tn, sialosyl-Tn, and T antigens in human colon cancer. Cancer Res. 1989 Jan 1;49(1):197–204. [PubMed] [Google Scholar]
- Kijima-Suda I., Miyamoto Y., Toyoshima S., Itoh M., Osawa T. Inhibition of experimental pulmonary metastasis of mouse colon adenocarcinoma 26 sublines by a sialic acid:nucleoside conjugate having sialyltransferase inhibiting activity. Cancer Res. 1986 Feb;46(2):858–862. [PubMed] [Google Scholar]
- Knibbs R. N., Goldstein I. J., Ratcliffe R. M., Shibuya N. Characterization of the carbohydrate binding specificity of the leukoagglutinating lectin from Maackia amurensis. Comparison with other sialic acid-specific lectins. J Biol Chem. 1991 Jan 5;266(1):83–88. [PubMed] [Google Scholar]
- Kojima H., Tsuchiya S., Sekiguchi K., Gelinas R., Hakomori S. Predefined gene transfer for expression of a glycosphingolipid antigen by transfection with a cosmid genomic library prepared from a cell line in which the specific glycosphingolipid is highly expressed. Biochem Biophys Res Commun. 1987 Mar 13;143(2):716–722. doi: 10.1016/0006-291x(87)91413-6. [DOI] [PubMed] [Google Scholar]
- Koprowski H., Steplewski Z., Mitchell K., Herlyn M., Herlyn D., Fuhrer P. Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet. 1979 Nov;5(6):957–971. doi: 10.1007/BF01542654. [DOI] [PubMed] [Google Scholar]
- Lackie P. M., Zuber C., Roth J. Polysialic acid and N-CAM localisation in embryonic rat kidney: mesenchymal and epithelial elements show different patterns of expression. Development. 1990 Nov;110(3):933–947. doi: 10.1242/dev.110.3.933. [DOI] [PubMed] [Google Scholar]
- Lance P., Lau K. M., Lau J. T. Isolation and characterization of a partial cDNA for a human sialyltransferase. Biochem Biophys Res Commun. 1989 Oct 16;164(1):225–232. doi: 10.1016/0006-291x(89)91706-3. [DOI] [PubMed] [Google Scholar]
- Lee E. U., Roth J., Paulson J. C. Alteration of terminal glycosylation sequences on N-linked oligosaccharides of Chinese hamster ovary cells by expression of beta-galactoside alpha 2,6-sialyltransferase. J Biol Chem. 1989 Aug 15;264(23):13848–13855. [PubMed] [Google Scholar]
- Lindholm L., Holmgren J., Svennerholm L., Fredman P., Nilsson O., Persson B., Myrvold H., Lagergård T. Monoclonal antibodies against gastrointestinal tumour-associated antigens isolated as monosialogangliosides. Int Arch Allergy Appl Immunol. 1983;71(2):178–181. doi: 10.1159/000233384. [DOI] [PubMed] [Google Scholar]
- Lucocq J. M., Roth J. Applications of immunocolloids in light microscopy. III. Demonstration of antigenic and lectin-binding sites in semithin resin sections. J Histochem Cytochem. 1984 Oct;32(10):1075–1083. doi: 10.1177/32.10.6384362. [DOI] [PubMed] [Google Scholar]
- Magnani J. L., Nilsson B., Brockhaus M., Zopf D., Steplewski Z., Koprowski H., Ginsburg V. A monoclonal antibody-defined antigen associated with gastrointestinal cancer is a ganglioside containing sialylated lacto-N-fucopentaose II. J Biol Chem. 1982 Dec 10;257(23):14365–14369. [PubMed] [Google Scholar]
- Morgenthaler J., Kemmner W., Brossmer R. Sialic acid dependent cell adhesion to collagen IV correlates with in vivo tumorigenicity of the human colon carcinoma sublines HCT116, HCT116a and HCT116b. Biochem Biophys Res Commun. 1990 Sep 14;171(2):860–866. doi: 10.1016/0006-291x(90)91225-h. [DOI] [PubMed] [Google Scholar]
- Nakasaki H., Mitomi T., Noto T., Ogoshi K., Hanaue H., Tanaka Y., Makuuchi H., Clausen H., Hakomori S. Mosaicism in the expression of tumor-associated carbohydrate antigens in human colonic and gastric cancers. Cancer Res. 1989 Jul 1;49(13):3662–3669. [PubMed] [Google Scholar]
- Nicolson G. L. The interactions of lectins with animal cell surfaces. Int Rev Cytol. 1974;39:89–190. doi: 10.1016/s0074-7696(08)60939-0. [DOI] [PubMed] [Google Scholar]
- Nilsson O., Månsson J. E., Lindholm L., Holmgren J., Svennerholm L. Sialosyllactotetraosylceramide, a novel ganglioside antigen detected in human carcinomas by a monoclonal antibody. FEBS Lett. 1985 Mar 25;182(2):398–402. doi: 10.1016/0014-5793(85)80341-0. [DOI] [PubMed] [Google Scholar]
- Nudelman E. D., Mandel U., Levery S. B., Kaizu T., Hakomori S. A series of disialogangliosides with binary 2----3 sialosyllactosamine structure, defined by monoclonal antibody NUH2, are oncodevelopmentally regulated antigens. J Biol Chem. 1989 Nov 5;264(31):18719–18725. [PubMed] [Google Scholar]
- O'Hanlon T. P., Lau K. M., Wang X. C., Lau J. T. Tissue-specific expression of beta-galactoside alpha-2,6-sialyltransferase. Transcript heterogeneity predicts a divergent polypeptide. J Biol Chem. 1989 Oct 15;264(29):17389–17394. [PubMed] [Google Scholar]
- Orntoft T. F., Harving N., Langkilde N. C. O-linked mucin-type glycoproteins in normal and malignant colon mucosa: lack of T-antigen expression and accumulation of Tn and sialosyl-Tn antigens in carcinomas. Int J Cancer. 1990 Apr 15;45(4):666–672. doi: 10.1002/ijc.2910450416. [DOI] [PubMed] [Google Scholar]
- Paulson J. C., Colley K. J. Glycosyltransferases. Structure, localization, and control of cell type-specific glycosylation. J Biol Chem. 1989 Oct 25;264(30):17615–17618. [PubMed] [Google Scholar]
- Paulson J. C., Weinstein J., Schauer A. Tissue-specific expression of sialyltransferases. J Biol Chem. 1989 Jul 5;264(19):10931–10934. [PubMed] [Google Scholar]
- Phillips M. L., Nudelman E., Gaeta F. C., Perez M., Singhal A. K., Hakomori S., Paulson J. C. ELAM-1 mediates cell adhesion by recognition of a carbohydrate ligand, sialyl-Lex. Science. 1990 Nov 23;250(4984):1130–1132. doi: 10.1126/science.1701274. [DOI] [PubMed] [Google Scholar]
- Rajan V. P., Larsen R. D., Ajmera S., Ernst L. K., Lowe J. B. A cloned human DNA restriction fragment determines expression of a GDP-L-fucose: beta-D-galactoside 2-alpha-L-fucosyltransferase in transfected cells. Evidence for isolation and transfer of the human H blood group locus. J Biol Chem. 1989 Jul 5;264(19):11158–11167. [PubMed] [Google Scholar]
- Ravindranath M. H., Higa H. H., Cooper E. L., Paulson J. C. Purification and characterization of an O-acetylsialic acid-specific lectin from a marine crab Cancer antennarius. J Biol Chem. 1985 Jul 25;260(15):8850–8856. [PubMed] [Google Scholar]
- Rettig W. J., Cordon-Cardo C., Ng J. S., Oettgen H. F., Old L. J., Lloyd K. O. High-molecular-weight glycoproteins of human teratocarcinoma defined by monoclonal antibodies to carbohydrate determinants. Cancer Res. 1985 Feb;45(2):815–821. [PubMed] [Google Scholar]
- Rogers G. N., Daniels R. S., Skehel J. J., Wiley D. C., Wang X. F., Higa H. H., Paulson J. C. Host-mediated selection of influenza virus receptor variants. Sialic acid-alpha 2,6Gal-specific clones of A/duck/Ukraine/1/63 revert to sialic acid-alpha 2,3Gal-specific wild type in ovo. J Biol Chem. 1985 Jun 25;260(12):7362–7367. [PubMed] [Google Scholar]
- Rogers G. N., Herrler G., Paulson J. C., Klenk H. D. Influenza C virus uses 9-O-acetyl-N-acetylneuraminic acid as a high affinity receptor determinant for attachment to cells. J Biol Chem. 1986 May 5;261(13):5947–5951. [PubMed] [Google Scholar]
- Rosenwald A. G., Stanley P., Krag S. S. Control of carbohydrate processing: increased beta-1,6 branching in N-linked carbohydrates of Lec9 CHO mutants appears to arise from a defect in oligosaccharide-dolichol biosynthesis. Mol Cell Biol. 1989 Mar;9(3):914–924. doi: 10.1128/mcb.9.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J. Distribution of concanavalin a receptors on normal rat liver cells and zajdela ascites hepatoma cells. Int J Cancer. 1974 Dec 15;14(6):762–770. doi: 10.1002/ijc.2910140610. [DOI] [PubMed] [Google Scholar]
- Roth J., Meyer H. W., Bolck F., Stiller D. Electron microscopic visualization of carbohydrate components in the glycocalyx of tumor cells using concanavalin A. Exp Pathol (Jena) 1972;6(3):189–192. [PubMed] [Google Scholar]
- Roth J., Neupert G., Bolck F. Concanavalin A receptors in the plasma membrane of rat liver cells: comparative electron microscopic studies on normal cells and on cells in vivo transformed by diethylnitrosamine. Exp Pathol (Jena) 1975;10(3-4):143–155. doi: 10.1016/s0014-4908(75)80018-4. [DOI] [PubMed] [Google Scholar]
- Roth J. Postembedding labeling on Lowicryl K4M tissue sections: detection and modification of cellular components. Methods Cell Biol. 1989;31:513–551. doi: 10.1016/s0091-679x(08)61625-8. [DOI] [PubMed] [Google Scholar]
- Rutishauser U., Watanabe M., Silver J., Troy F. A., Vimr E. R. Specific alteration of NCAM-mediated cell adhesion by an endoneuraminidase. J Cell Biol. 1985 Nov;101(5 Pt 1):1842–1849. doi: 10.1083/jcb.101.5.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sariola H., Aufderheide E., Bernhard H., Henke-Fahle S., Dippold W., Ekblom P. Antibodies to cell surface ganglioside GD3 perturb inductive epithelial-mesenchymal interactions. Cell. 1988 Jul 15;54(2):235–245. doi: 10.1016/0092-8674(88)90556-9. [DOI] [PubMed] [Google Scholar]
- Sata T., Lackie P. M., Taatjes D. J., Peumans W., Roth J. Detection of the Neu5 Ac (alpha 2,3) Gal (beta 1,4) GlcNAc sequence with the leukoagglutinin from Maackia amurensis: light and electron microscopic demonstration of differential tissue expression of terminal sialic acid in alpha 2,3- and alpha 2,6-linkage. J Histochem Cytochem. 1989 Nov;37(11):1577–1588. doi: 10.1177/37.11.2478613. [DOI] [PubMed] [Google Scholar]
- Sata T., Zuber C., Roth J. Lectin--digoxigenin conjugates: a new hapten system for glycoconjugate cytochemistry. Histochemistry. 1990;94(1):1–11. doi: 10.1007/BF00266783. [DOI] [PubMed] [Google Scholar]
- Schauer R. Chemistry, metabolism, and biological functions of sialic acids. Adv Carbohydr Chem Biochem. 1982;40:131–234. doi: 10.1016/s0065-2318(08)60109-2. [DOI] [PubMed] [Google Scholar]
- Shibuya N., Goldstein I. J., Broekaert W. F., Nsimba-Lubaki M., Peeters B., Peumans W. J. The elderberry (Sambucus nigra L.) bark lectin recognizes the Neu5Ac(alpha 2-6)Gal/GalNAc sequence. J Biol Chem. 1987 Feb 5;262(4):1596–1601. [PubMed] [Google Scholar]
- Skutelsky E., Goyal V., Alroy J. The use of avidin-gold complex for light microscopic localization of lectin receptors. Histochemistry. 1987;86(3):291–295. doi: 10.1007/BF00490261. [DOI] [PubMed] [Google Scholar]
- Slot J. W., Geuze H. J. A new method of preparing gold probes for multiple-labeling cytochemistry. Eur J Cell Biol. 1985 Jul;38(1):87–93. [PubMed] [Google Scholar]
- Stoolman L. M. Adhesion molecules controlling lymphocyte migration. Cell. 1989 Mar 24;56(6):907–910. doi: 10.1016/0092-8674(89)90620-x. [DOI] [PubMed] [Google Scholar]
- Taatjes D. J., Roth J. Glycosylation in intestinal epithelium. Int Rev Cytol. 1991;126:135–193. doi: 10.1016/S0074-7696(08)60684-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taatjes D. J., Roth J., Peumans W., Goldstein I. J. Elderberry bark lectin--gold techniques for the detection of Neu5Ac (alpha 2,6) Gal/GalNAc sequences: applications and limitations. Histochem J. 1988 Sep;20(9):478–490. doi: 10.1007/BF01002646. [DOI] [PubMed] [Google Scholar]
- True D. D., Singer M. S., Lasky L. A., Rosen S. D. Requirement for sialic acid on the endothelial ligand of a lymphocyte homing receptor. J Cell Biol. 1990 Dec;111(6 Pt 1):2757–2764. doi: 10.1083/jcb.111.6.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner H. E., Thomas P., Wolf B. C., Rapoza A., Steele G., Jr Inhibition of sialic acid incorporation prevents hepatic metastases. Arch Surg. 1990 Mar;125(3):351–354. doi: 10.1001/archsurg.1990.01410150073013. [DOI] [PubMed] [Google Scholar]
- Wang W. C., Cummings R. D. The immobilized leukoagglutinin from the seeds of Maackia amurensis binds with high affinity to complex-type Asn-linked oligosaccharides containing terminal sialic acid-linked alpha-2,3 to penultimate galactose residues. J Biol Chem. 1988 Apr 5;263(10):4576–4585. [PubMed] [Google Scholar]
- Wolf B. C., Salem R. R., Sears H. F., Horst D. A., Lavin P. T., Herlyn M., Itzkowitz S. H., Schlom J., Steele G. D., Jr The expression of colorectal carcinoma-associated antigens in the normal colonic mucosa. An immunohistochemical analysis of regional distribution. Am J Pathol. 1989 Jul;135(1):111–119. [PMC free article] [PubMed] [Google Scholar]
- Yagel S., Feinmesser R., Waghorne C., Lala P. K., Breitman M. L., Dennis J. W. Evidence that beta 1-6 branched Asn-linked oligosaccharides on metastatic tumor cells facilitate invasion of basement membranes. Int J Cancer. 1989 Oct 15;44(4):685–690. doi: 10.1002/ijc.2910440422. [DOI] [PubMed] [Google Scholar]
- Yogeeswaran G., Salk P. L. Metastatic potential is positively correlated with cell surface sialylation of cultured murine tumor cell lines. Science. 1981 Jun 26;212(4502):1514–1516. doi: 10.1126/science.7233237. [DOI] [PubMed] [Google Scholar]