Abstract
Human atherogenesis is a pleiotropic process with an undefined cause. Several pathologic factors have been linked to the disease process, including arterial injury or activation of the endothelium, which may injury or activation of the endothelium, which may initiate proatherosclerotic events in the vessel wall. Atherosclerotic lesions are characterized, in part, by the presence of activated immune cells, abnormal cell proliferation, and altered cholesterol metabolism. These activated immunocompetent cells in plaques produce vasoactive mediators that can alter homeostasis and may promote the arteriopathy. Both molecular and structural evidence is presented that herpesviruses, by way of induction of altered gene function and cellular cholesterol metabolism, coupled with their ability to activate coagulation and a monocyte receptor on the infected endothelium, are involved in major pathogenic events associated with atherosclerosis and thrombosis. Work from the author's laboratory, as well as from other research groups, have shown that avian and human herpesviruses act specifically to induce alterations to the surface and inner layers of the blood vessel wall that may predispose to atherosclerosis and its attendant clinical complications.
Full text
PDF


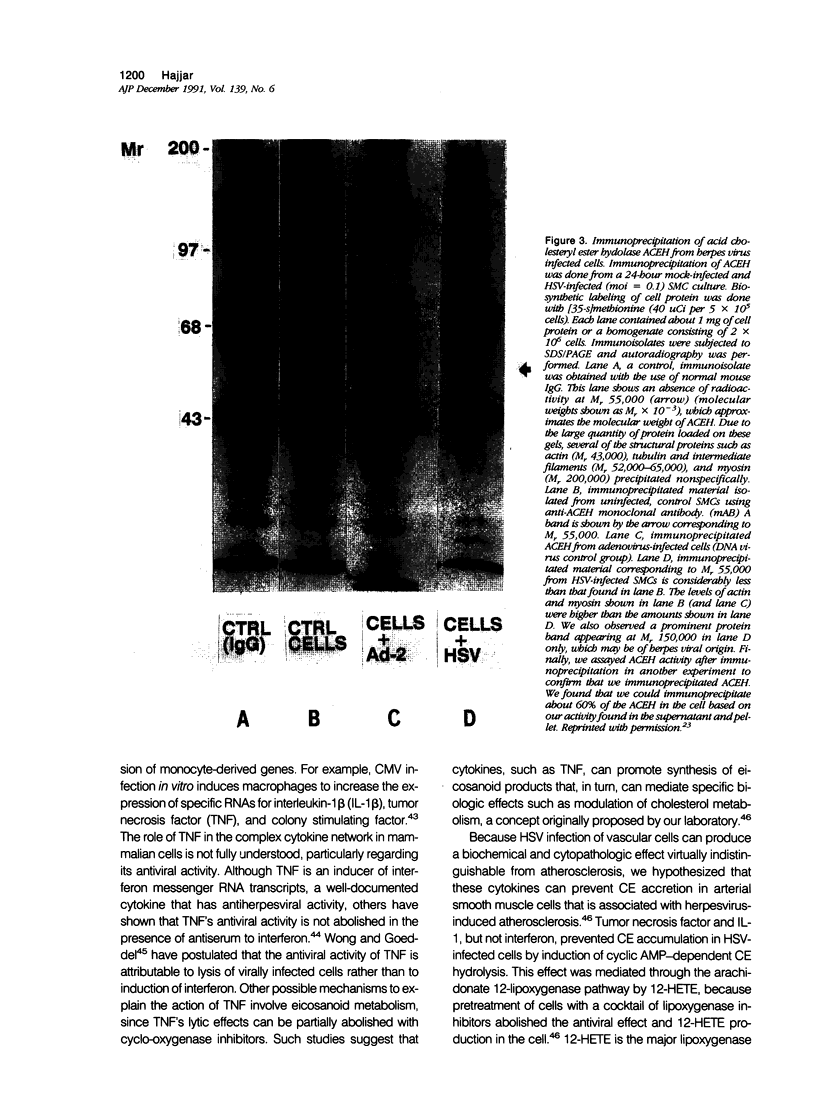
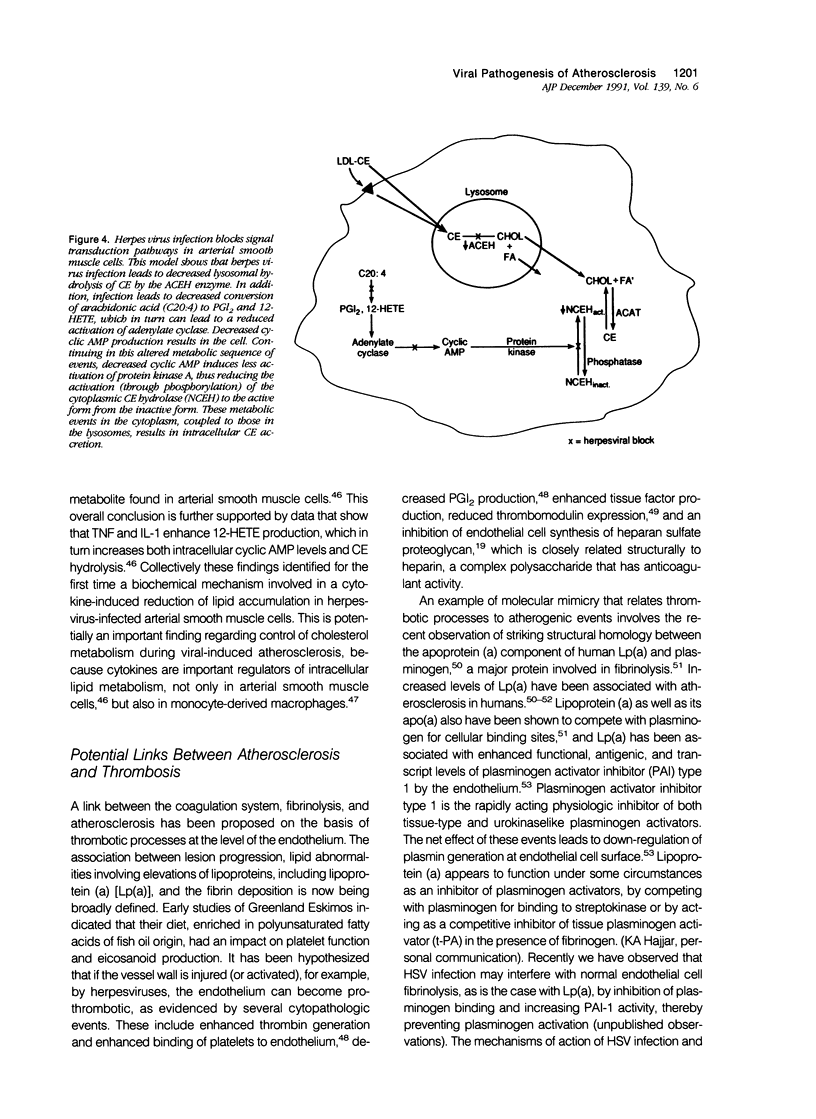

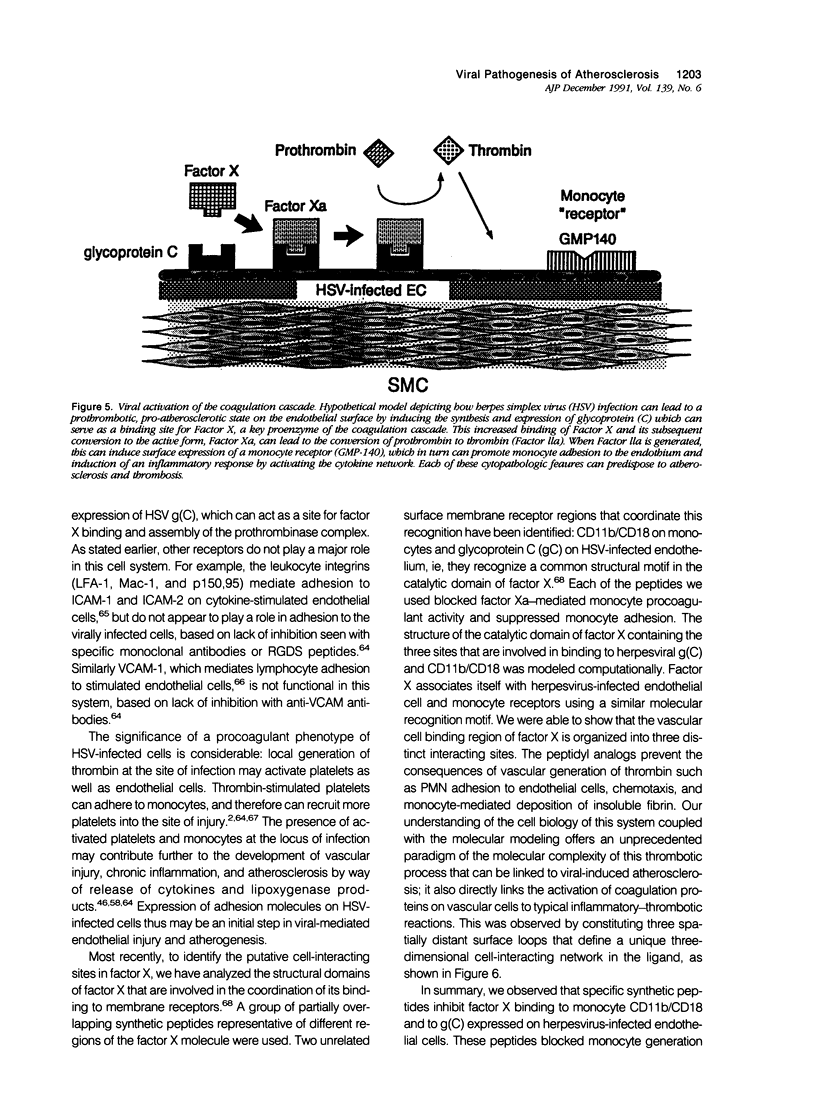


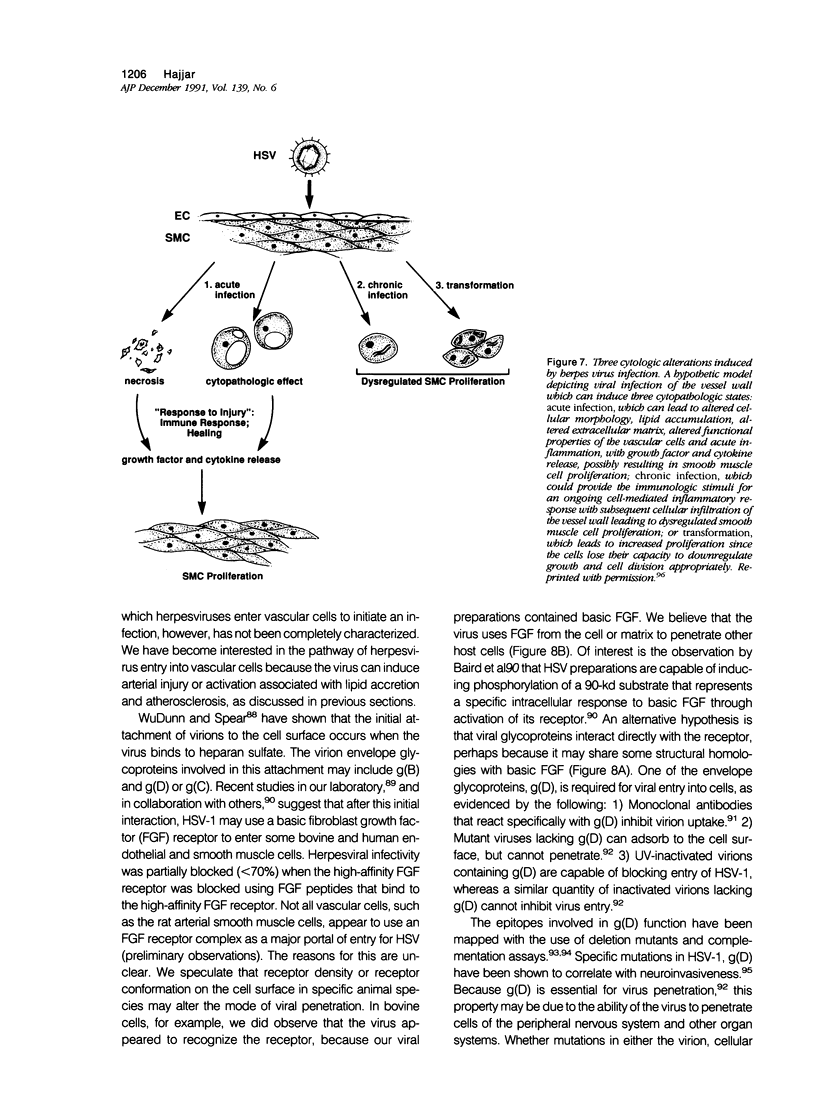




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam E., Melnick J. L., Probtsfield J. L., Petrie B. L., Burek J., Bailey K. R., McCollum C. H., DeBakey M. E. High levels of cytomegalovirus antibody in patients requiring vascular surgery for atherosclerosis. Lancet. 1987 Aug 8;2(8554):291–293. doi: 10.1016/s0140-6736(87)90888-9. [DOI] [PubMed] [Google Scholar]
- Baird A., Florkiewicz R. Z., Maher P. A., Kaner R. J., Hajjar D. P. Mediation of virion penetration into vascular cells by association of basic fibroblast growth factor with herpes simplex virus type 1. Nature. 1990 Nov 22;348(6299):344–346. doi: 10.1038/348344a0. [DOI] [PubMed] [Google Scholar]
- Benditt E. P., Barrett T., McDougall J. K. Viruses in the etiology of atherosclerosis. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6386–6389. doi: 10.1073/pnas.80.20.6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benditt E. P., Benditt J. M. Evidence for a monoclonal origin of human atherosclerotic plaques. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1753–1756. doi: 10.1073/pnas.70.6.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Majeau G. R., Fiers W., Cotran R. S., Gimbrone M. A., Jr Recombinant tumor necrosis factor induces procoagulant activity in cultured human vascular endothelium: characterization and comparison with the actions of interleukin 1. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4533–4537. doi: 10.1073/pnas.83.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Mendrick D. L., Cotran R. S., Gimbrone M. A., Jr Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron I. R., Park M., Dutia B. M., Orr A., Macnab J. C. Herpes simplex virus sequences involved in the initiation of oncogenic morphological transformation of rat cells are not required for maintenance of the transformed state. J Gen Virol. 1985 Mar;66(Pt 3):517–527. doi: 10.1099/0022-1317-66-3-517. [DOI] [PubMed] [Google Scholar]
- Chen J. K., Li L., McClure D. B. Altered low density lipoprotein receptor regulation is associated with cholesteryl ester accumulation in Simian virus 40 transformed rodent fibroblast cell lines. In Vitro Cell Dev Biol. 1988 Apr;24(4):353–358. doi: 10.1007/BF02628838. [DOI] [PubMed] [Google Scholar]
- Cines D. B., Lyss A. P., Bina M., Corkey R., Kefalides N. A., Friedman H. M. Fc and C3 receptors induced by herpes simplex virus on cultured human endothelial cells. J Clin Invest. 1982 Jan;69(1):123–128. doi: 10.1172/JCI110422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudding L., Haskill S., Clark B. D., Auron P. E., Sporn S., Huang E. S. Cytomegalovirus infection stimulates expression of monocyte-associated mediator genes. J Immunol. 1989 Nov 15;143(10):3343–3352. [PubMed] [Google Scholar]
- Etingin O. R., Hajjar D. P. Evidence for cytokine regulation of cholesterol metabolism in herpesvirus-infected arterial cells by the lipoxygenase pathway. J Lipid Res. 1990 Feb;31(2):299–305. [PubMed] [Google Scholar]
- Etingin O. R., Hajjar D. P., Hajjar K. A., Harpel P. C., Nachman R. L. Lipoprotein (a) regulates plasminogen activator inhibitor-1 expression in endothelial cells. A potential mechanism in thrombogenesis. J Biol Chem. 1991 Feb 5;266(4):2459–2465. [PubMed] [Google Scholar]
- Etingin O. R., Silverstein R. L., Friedman H. M., Hajjar D. P. Viral activation of the coagulation cascade: molecular interactions at the surface of infected endothelial cells. Cell. 1990 May 18;61(4):657–662. doi: 10.1016/0092-8674(90)90477-v. [DOI] [PubMed] [Google Scholar]
- Etingin O. R., Silverstein R. L., Hajjar D. P. Identification of a monocyte receptor on herpesvirus-infected endothelial cells. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7200–7203. doi: 10.1073/pnas.88.16.7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabricant C. G., Fabricant J., Litrenta M. M., Minick C. R. Virus-induced atherosclerosis. J Exp Med. 1978 Jul 1;148(1):335–340. doi: 10.1084/jem.148.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabricant C. G., Fabricant J., Minick C. R., Litrenta M. M. Herpesvirus-induced atherosclerosis in chickens. Fed Proc. 1983 May 15;42(8):2476–2479. [PubMed] [Google Scholar]
- Fabricant C. G., Krook L., Gillespie J. H. Virus-induced cholesterol crystals. Science. 1973 Aug 10;181(4099):566–567. doi: 10.1126/science.181.4099.566. [DOI] [PubMed] [Google Scholar]
- Fenwick M. L., Clark J. Early and delayed shut-off of host protein synthesis in cells infected with herpes simplex virus. J Gen Virol. 1982 Jul;61(Pt 50):121–125. doi: 10.1099/0022-1317-61-1-121. [DOI] [PubMed] [Google Scholar]
- Frenkel N., Schirmer E. C., Wyatt L. S., Katsafanas G., Roffman E., Danovich R. M., June C. H. Isolation of a new herpesvirus from human CD4+ T cells. Proc Natl Acad Sci U S A. 1990 Jan;87(2):748–752. doi: 10.1073/pnas.87.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman H. M., Cohen G. H., Eisenberg R. J., Seidel C. A., Cines D. B. Glycoprotein C of herpes simplex virus 1 acts as a receptor for the C3b complement component on infected cells. Nature. 1984 Jun 14;309(5969):633–635. doi: 10.1038/309633a0. [DOI] [PubMed] [Google Scholar]
- Friedman H. M., Macarak E. J., MacGregor R. R., Wolfe J., Kefalides N. A. Virus infection of endothelial cells. J Infect Dis. 1981 Feb;143(2):266–273. doi: 10.1093/infdis/143.2.266. [DOI] [PubMed] [Google Scholar]
- Fuller A. O., Spear P. G. Anti-glycoprotein D antibodies that permit adsorption but block infection by herpes simplex virus 1 prevent virion-cell fusion at the cell surface. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5454–5458. doi: 10.1073/pnas.84.15.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs C. P., Nazerian K., Velicer L. F., Kung H. J. Extensive homology exists between Marek disease herpesvirus and its vaccine virus, herpesvirus of turkeys. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3365–3369. doi: 10.1073/pnas.81.11.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good D. J., Polverini P. J., Rastinejad F., Le Beau M. M., Lemons R. S., Frazier W. A., Bouck N. P. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6624–6628. doi: 10.1073/pnas.87.17.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon T., Garcia-Palmieri M. R., Kagan A., Kannel W. B., Schiffman J. Differences in coronary heart disease in Framingham, Honolulu and Puerto Rico. J Chronic Dis. 1974 Sep;27(7-8):329–344. doi: 10.1016/0021-9681(74)90013-7. [DOI] [PubMed] [Google Scholar]
- Grattan M. T., Moreno-Cabral C. E., Starnes V. A., Oyer P. E., Stinson E. B., Shumway N. E. Cytomegalovirus infection is associated with cardiac allograft rejection and atherosclerosis. JAMA. 1989 Jun 23;261(24):3561–3566. [PubMed] [Google Scholar]
- Gyorkey F., Melnick J. L., Guinn G. A., Gyorkey P., DeBakey M. E. Herpesviridae in the endothelial and smooth muscle cells of the proximal aorta in arteriosclerotic patients. Exp Mol Pathol. 1984 Jun;40(3):328–339. doi: 10.1016/0014-4800(84)90050-9. [DOI] [PubMed] [Google Scholar]
- Hajjar D. P., Fabricant C. G., Minick C. R., Fabricant J. Virus-induced atherosclerosis. Herpesvirus infection alters aortic cholesterol metabolism and accumulation. Am J Pathol. 1986 Jan;122(1):62–70. [PMC free article] [PubMed] [Google Scholar]
- Hajjar D. P., Falcone D. J., Fabricant C. G., Fabricant J. Altered cholesteryl ester cycle is associated with lipid accumulation in herpesvirus-infected arterial smooth muscle cells. J Biol Chem. 1985 May 25;260(10):6124–6128. [PubMed] [Google Scholar]
- Hajjar D. P. Herpesvirus infection prevents activation of cytoplasmic cholesteryl esterase in arterial smooth muscle cells. J Biol Chem. 1986 Jun 15;261(17):7611–7614. [PubMed] [Google Scholar]
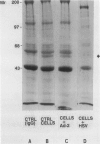
- Hajjar D. P., Nicholson A. C., Hajjar K. A., Sando G. N., Summers B. D. Decreased messenger RNA translation in herpesvirus-infected arterial cells: effects on cholesteryl ester hydrolase. Proc Natl Acad Sci U S A. 1989 May;86(9):3366–3370. doi: 10.1073/pnas.86.9.3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar D. P., Pomerantz K. B., Falcone D. J., Weksler B. B., Grant A. J. Herpes simplex virus infection in human arterial cells. Implications in arteriosclerosis. J Clin Invest. 1987 Nov;80(5):1317–1321. doi: 10.1172/JCI113208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar K. A., Gavish D., Breslow J. L., Nachman R. L. Lipoprotein(a) modulation of endothelial cell surface fibrinolysis and its potential role in atherosclerosis. Nature. 1989 May 25;339(6222):303–305. doi: 10.1038/339303a0. [DOI] [PubMed] [Google Scholar]
- Hattori R., Hamilton K. K., Fugate R. D., McEver R. P., Sims P. J. Stimulated secretion of endothelial von Willebrand factor is accompanied by rapid redistribution to the cell surface of the intracellular granule membrane protein GMP-140. J Biol Chem. 1989 May 15;264(14):7768–7771. [PubMed] [Google Scholar]
- Hendrix M. G., Salimans M. M., van Boven C. P., Bruggeman C. A. High prevalence of latently present cytomegalovirus in arterial walls of patients suffering from grade III atherosclerosis. Am J Pathol. 1990 Jan;136(1):23–28. [PMC free article] [PubMed] [Google Scholar]
- Herold B. C., WuDunn D., Soltys N., Spear P. G. Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J Virol. 1991 Mar;65(3):1090–1098. doi: 10.1128/jvi.65.3.1090-1098.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho D. D., Rota T. R., Andrews C. A., Hirsch M. S. Replication of human cytomegalovirus in endothelial cells. J Infect Dis. 1984 Dec;150(6):956–957. doi: 10.1093/infdis/150.6.956. [DOI] [PubMed] [Google Scholar]
- Hwang C. B., Shillitoe E. J. DNA sequence of mutations induced in cells by herpes simplex virus type-1. Virology. 1990 Sep;178(1):180–188. doi: 10.1016/0042-6822(90)90392-5. [DOI] [PubMed] [Google Scholar]
- Ishibashi S., Inaba T., Shimano H., Harada K., Inoue I., Mokuno H., Mori N., Gotoda T., Takaku F., Yamada N. Monocyte colony-stimulating factor enhances uptake and degradation of acetylated low density lipoproteins and cholesterol esterification in human monocyte-derived macrophages. J Biol Chem. 1990 Aug 25;265(24):14109–14117. [PubMed] [Google Scholar]
- Izumi K. M., Stevens J. G. Molecular and biological characterization of a herpes simplex virus type 1 (HSV-1) neuroinvasiveness gene. J Exp Med. 1990 Aug 1;172(2):487–496. doi: 10.1084/jem.172.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Frame M. C., Ligas M. W., Cross A. M., Stow N. D. Herpes simplex virus immunoglobulin G Fc receptor activity depends on a complex of two viral glycoproteins, gE and gI. J Virol. 1988 Apr;62(4):1347–1354. doi: 10.1128/jvi.62.4.1347-1354.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. E., Nahmias A. J., Magder L. S., Lee F. K., Brooks C. A., Snowden C. B. A seroepidemiologic survey of the prevalence of herpes simplex virus type 2 infection in the United States. N Engl J Med. 1989 Jul 6;321(1):7–12. doi: 10.1056/NEJM198907063210102. [DOI] [PubMed] [Google Scholar]
- Kaner R. J., Baird A., Mansukhani A., Basilico C., Summers B. D., Florkiewicz R. Z., Hajjar D. P. Fibroblast growth factor receptor is a portal of cellular entry for herpes simplex virus type 1. Science. 1990 Jun 15;248(4961):1410–1413. doi: 10.1126/science.2162560. [DOI] [PubMed] [Google Scholar]
- Kaner R. J., Iozzo R. V., Ziaie Z., Kefalides N. A. Inhibition of proteoglycan synthesis in human endothelial cells after infection with herpes simplex virus type 1 in vitro. Am J Respir Cell Mol Biol. 1990 May;2(5):423–431. doi: 10.1165/ajrcmb/2.5.423. [DOI] [PubMed] [Google Scholar]
- Kefalides N. A., Ziaie Z. Herpes simplex virus suppression of human endothelial matrix protein synthesis is independent of viral protein synthesis. Lab Invest. 1986 Sep;55(3):328–336. [PubMed] [Google Scholar]
- Key N. S., Vercellotti G. M., Winkelmann J. C., Moldow C. F., Goodman J. L., Esmon N. L., Esmon C. T., Jacob H. S. Infection of vascular endothelial cells with herpes simplex virus enhances tissue factor activity and reduces thrombomodulin expression. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7095–7099. doi: 10.1073/pnas.87.18.7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohase M., Henriksen-DeStefano D., May L. T., Vilcek J., Sehgal P. B. Induction of beta 2-interferon by tumor necrosis factor: a homeostatic mechanism in the control of cell proliferation. Cell. 1986 Jun 6;45(5):659–666. doi: 10.1016/0092-8674(86)90780-4. [DOI] [PubMed] [Google Scholar]
- Kwong A. D., Kruper J. A., Frenkel N. Herpes simplex virus virion host shutoff function. J Virol. 1988 Mar;62(3):912–921. doi: 10.1128/jvi.62.3.912-921.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder D., Gartler S. M. Glucose-6-phosphate dehydrogenase mosaicism: utilization as a cell marker in the study of leiomyomas. Science. 1965 Oct 1;150(3692):67–69. doi: 10.1126/science.150.3692.67. [DOI] [PubMed] [Google Scholar]
- London F. S., Brinker J. M., Ziaie Z., Kefalides N. A. Suppression of host mRNA in human smooth muscle cells by a virion competent factor in herpes simplex virus type 1. Lab Invest. 1990 Feb;62(2):189–195. [PubMed] [Google Scholar]
- MacGregor R. R., Friedman H. M., Macarak E. J., Kefalides N. A. Virus infection of endothelial cells increases granulocyte adherence. J Clin Invest. 1980 Jun;65(6):1469–1477. doi: 10.1172/JCI109811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnab J. C. Herpes simplex virus and human cytomegalovirus: their role in morphological transformation and genital cancers. J Gen Virol. 1987 Oct;68(Pt 10):2525–2550. doi: 10.1099/0022-1317-68-10-2525. [DOI] [PubMed] [Google Scholar]
- Majesky M. W., Reidy M. A., Benditt E. P., Juchau M. R. Focal smooth muscle proliferation in the aortic intima produced by an initiation-promotion sequence. Proc Natl Acad Sci U S A. 1985 May;82(10):3450–3454. doi: 10.1073/pnas.82.10.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald K., Rector T. S., Braulin E. A., Kubo S. H., Olivari M. T. Association of coronary artery disease in cardiac transplant recipients with cytomegalovirus infection. Am J Cardiol. 1989 Aug 1;64(5):359–362. doi: 10.1016/0002-9149(89)90535-3. [DOI] [PubMed] [Google Scholar]
- McEver R. P. GMP-140: a receptor for neutrophils and monocytes on activated platelets and endothelium. J Cell Biochem. 1991 Feb;45(2):156–161. doi: 10.1002/jcb.240450206. [DOI] [PubMed] [Google Scholar]
- Melnick J. L., Petrie B. L., Dreesman G. R., Burek J., McCollum C. H., DeBakey M. E. Cytomegalovirus antigen within human arterial smooth muscle cells. Lancet. 1983 Sep 17;2(8351):644–647. doi: 10.1016/s0140-6736(83)92529-1. [DOI] [PubMed] [Google Scholar]
- Minick C. R., Fabricant C. G., Fabricant J., Litrenta M. M. Atheroarteriosclerosis induced by infection with a herpesvirus. Am J Pathol. 1979 Sep;96(3):673–706. [PMC free article] [PubMed] [Google Scholar]
- Muggeridge M. I., Isola V. J., Byrn R. A., Tucker T. J., Minson A. C., Glorioso J. C., Cohen G. H., Eisenberg R. J. Antigenic analysis of a major neutralization site of herpes simplex virus glycoprotein D, using deletion mutants and monoclonal antibody-resistant mutants. J Virol. 1988 Sep;62(9):3274–3280. doi: 10.1128/jvi.62.9.3274-3280.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muggeridge M. I., Wilcox W. C., Cohen G. H., Eisenberg R. J. Identification of a site on herpes simplex virus type 1 glycoprotein D that is essential for infectivity. J Virol. 1990 Aug;64(8):3617–3626. doi: 10.1128/jvi.64.8.3617-3626.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachtigal M., Legrand A., Greenspan P., Nachtigal S. A., Nagpal M. L. Immortalization of rabbit vascular smooth muscle cells after transfection with a fragment of the BglII N region of herpes simplex virus type 2 DNA. Intervirology. 1990;31(2-4):166–174. doi: 10.1159/000150151. [DOI] [PubMed] [Google Scholar]
- Nachtigal M., Legrand A., Nagpal M. L., Nachtigal S. A., Greenspan P. Transformation of rabbit vascular smooth muscle cells by transfection with the early region of SV40 DNA. Am J Pathol. 1990 Feb;136(2):297–306. [PMC free article] [PubMed] [Google Scholar]
- Nishioka Y., Silverstein S. Degradation of cellular mRNA during infection by herpes simplex virus. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2370–2374. doi: 10.1073/pnas.74.6.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn L., Hession C., Tizard R., Vassallo C., Luhowskyj S., Chi-Rosso G., Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989 Dec 22;59(6):1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- Para M. F., Baucke R. B., Spear P. G. Glycoprotein gE of herpes simplex virus type 1: effects of anti-gE on virion infectivity and on virus-induced fc-binding receptors. J Virol. 1982 Jan;41(1):129–136. doi: 10.1128/jvi.41.1.129-136.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes J. L., Cardell R. R., Hubbard F. C., Jr, Hubbard D., Meltzer A., Penn A. Cultured human atherosclerotic plaque smooth muscle cells retain transforming potential and display enhanced expression of the myc protooncogene. Am J Pathol. 1991 Mar;138(3):765–775. [PMC free article] [PubMed] [Google Scholar]
- Pearson T. A., Wang B. A., Solez K., Heptinstall R. H. Clonal characteristics of fibrous plaques and fatty streaks from human aortas. Am J Pathol. 1975 Nov;81(2):379–387. [PMC free article] [PubMed] [Google Scholar]
- Penn A., Garte S. J., Warren L., Nesta D., Mindich B. Transforming gene in human atherosclerotic plaque DNA. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7951–7955. doi: 10.1073/pnas.83.20.7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn A., Snyder C. Arteriosclerotic plaque development is 'promoted' by polynuclear aromatic hydrocarbons. Carcinogenesis. 1988 Dec;9(12):2185–2189. doi: 10.1093/carcin/9.12.2185. [DOI] [PubMed] [Google Scholar]
- Petrie B. L., Melnick J. L., Adam E., Burek J., McCollum C. H., DeBakey M. E. Nucleic acid sequences of cytomegalovirus in cells cultured from human arterial tissue. J Infect Dis. 1987 Jan;155(1):158–159. doi: 10.1093/infdis/155.1.158. [DOI] [PubMed] [Google Scholar]
- Pomerantz K. B., Hajjar D. P. Eicosanoids in regulation of arterial smooth muscle cell phenotype, proliferative capacity, and cholesterol metabolism. Arteriosclerosis. 1989 Jul-Aug;9(4):413–429. doi: 10.1161/01.atv.9.4.413. [DOI] [PubMed] [Google Scholar]
- Rastinejad F., Polverini P. J., Bouck N. P. Regulation of the activity of a new inhibitor of angiogenesis by a cancer suppressor gene. Cell. 1989 Feb 10;56(3):345–355. doi: 10.1016/0092-8674(89)90238-9. [DOI] [PubMed] [Google Scholar]
- Ross R. George Lyman Duff Memorial Lecture. Atherosclerosis: a problem of the biology of arterial wall cells and their interactions with blood components. Arteriosclerosis. 1981 Sep-Oct;1(5):293–311. doi: 10.1161/01.atv.1.5.293. [DOI] [PubMed] [Google Scholar]
- Sanchez-Madrid F., Nagy J. A., Robbins E., Simon P., Springer T. A. A human leukocyte differentiation antigen family with distinct alpha-subunits and a common beta-subunit: the lymphocyte function-associated antigen (LFA-1), the C3bi complement receptor (OKM1/Mac-1), and the p150,95 molecule. J Exp Med. 1983 Dec 1;158(6):1785–1803. doi: 10.1084/jem.158.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S. M., Campbell G. R., Campbell J. H. Replication of smooth muscle cells in vascular disease. Circ Res. 1986 Apr;58(4):427–444. doi: 10.1161/01.res.58.4.427. [DOI] [PubMed] [Google Scholar]
- Silverstein R. L., Nachman R. L. Thrombospondin binds to monocytes-macrophages and mediates platelet-monocyte adhesion. J Clin Invest. 1987 Mar;79(3):867–874. doi: 10.1172/JCI112896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus S. E., Rooney J. F., Sever J. L., Seidlin M., Nusinoff-Lehrman S., Cremer K. NIH Conference. Herpes simplex virus infection: biology, treatment, and prevention. Ann Intern Med. 1985 Sep;103(3):404–419. doi: 10.7326/0003-4819-103-3-404. [DOI] [PubMed] [Google Scholar]
- Sydiskis R. J., Roizman B. Polysomes and protein synthesis in cells infected with a DNA virus. Science. 1966 Jul 1;153(3731):76–78. doi: 10.1126/science.153.3731.76. [DOI] [PubMed] [Google Scholar]
- Thomas W. A., Kim D. N. Biology of disease. Atherosclerosis as a hyperplastic and/or neoplastic process. Lab Invest. 1983 Mar;48(3):245–255. [PubMed] [Google Scholar]
- Thomas W. A., Reiner J. M., Janakidevi K., Florentin R. A., Lee K. T. Population dynamics of arterial cells during atherogenesis. X. Study of monotypism in atherosclerotic lesions of black women heterozygous for glucose-6-phosphate dehydrogenase (G-6-PD). Exp Mol Pathol. 1979 Dec;31(3):367–386. doi: 10.1016/0014-4800(79)90038-8. [DOI] [PubMed] [Google Scholar]
- Tumilowicz J. J., Gawlik M. E., Powell B. B., Trentin J. J. Replication of cytomegalovirus in human arterial smooth muscle cells. J Virol. 1985 Dec;56(3):839–845. doi: 10.1128/jvi.56.3.839-845.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercellotti G. M. Proinflammatory and procoagulant effects of herpes simplex infection on human endothelium. Blood Cells. 1990;16(1):209–216. [PubMed] [Google Scholar]
- Visser M. R., Jacob H. S., Goodman J. L., McCarthy J. B., Furcht L. T., Vercellotti G. M. Granulocyte-mediated injury to herpes simplex virus-infected human endothelium. Lab Invest. 1989 Feb;60(2):296–304. [PubMed] [Google Scholar]
- Visser M. R., Tracy P. B., Vercellotti G. M., Goodman J. L., White J. G., Jacob H. S. Enhanced thrombin generation and platelet binding on herpes simplex virus-infected endothelium. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8227–8230. doi: 10.1073/pnas.85.21.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton K. W., Hitchens J., Magnani H. N., Khan M. A study of methods of identification and estimation of Lp(a) lipoprotein and of its significance in health, hyperlipidaemia and atherosclerosis. Atherosclerosis. 1974 Sep-Oct;20(2):323–346. doi: 10.1016/0021-9150(74)90016-1. [DOI] [PubMed] [Google Scholar]
- Wong G. H., Goeddel D. V. Tumour necrosis factors alpha and beta inhibit virus replication and synergize with interferons. 1986 Oct 30-Nov 5Nature. 323(6091):819–822. doi: 10.1038/323819a0. [DOI] [PubMed] [Google Scholar]
- WuDunn D., Spear P. G. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol. 1989 Jan;63(1):52–58. doi: 10.1128/jvi.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiroya H. M., Ghosh L., Yang R., Robertson A. L., Jr Herpesviridae in the coronary arteries and aorta of young trauma victims. Am J Pathol. 1988 Jan;130(1):71–79. [PMC free article] [PubMed] [Google Scholar]
- Yew P. R., Rajavashisth T. B., Forrester J., Barath P., Lusis A. J. NIH3T3 transforming gene not a general feature of atherosclerotic plaque DNA (atherosclerosis/oncogene/NIH3T3 transfection assay). Biochem Biophys Res Commun. 1989 Dec 29;165(3):1067–1071. doi: 10.1016/0006-291x(89)92710-1. [DOI] [PubMed] [Google Scholar]