Abstract
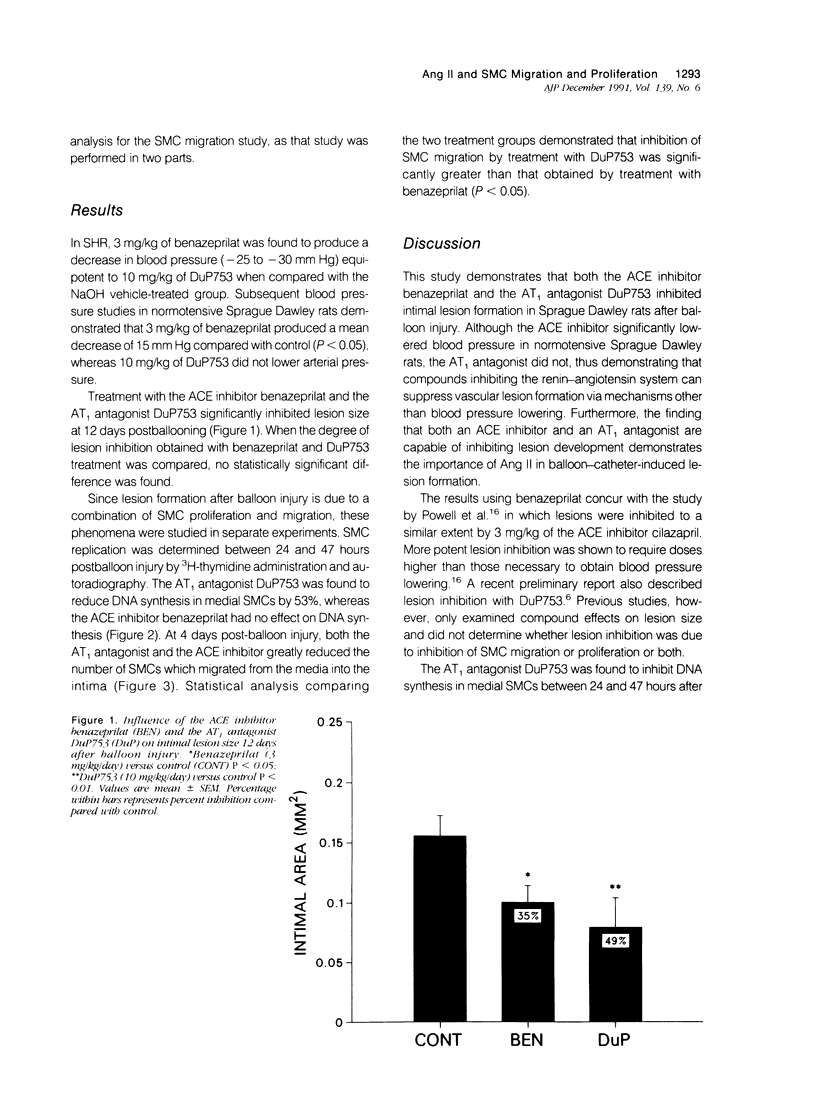
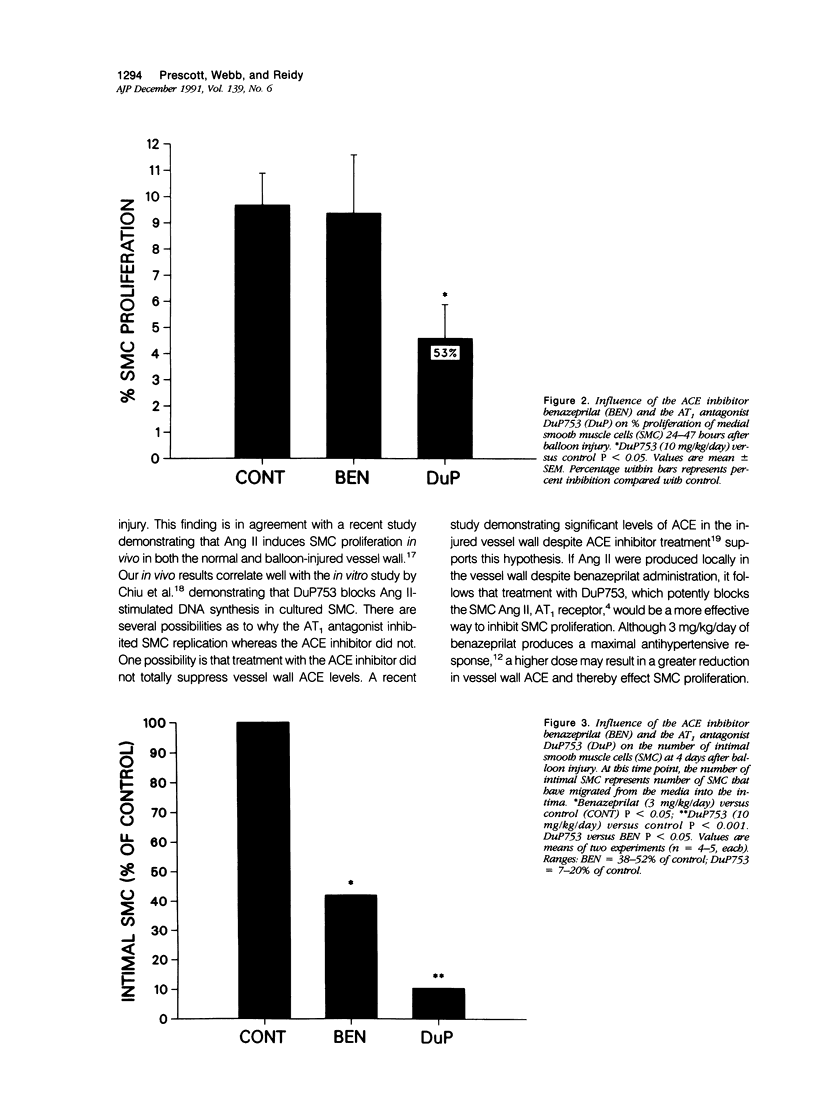
The angiotensin-converting enzyme (ACE) inhibitor, benazeprilat and the angiotensin II (Ang II), AT1-specific receptor antagonist, DuP753, were compared for their effects on intimal lesion formation as well as smooth muscle cell (SMC) proliferation and migration in Sprague Dawley rats after carotid balloon injury. Both the ACE inhibitor (benazeprilat, 3 mg/kg/day) and the AT1 antagonist (DuP 753, 10 mg/kg/day) significantly reduced intimal lesion formation after balloon injury (by 35% and 49%, respectively). Medial SMC proliferation after injury was reduced 53% by the AT1 antagonist; however, the ACE inhibitor had no effect on SMC proliferation. SMC migration was reduced 94% by the AT1 antagonist and 68% by the ACE inhibitor. These data demonstrate the importance of Ang II in SMC proliferation and migration after balloon injury. They also demonstrate that in the balloon injury model, the ACE inhibitor reduced intimal lesion size by inhibiting SMC migration alone without affecting SMC proliferation. A more pronounced reduction in lesion size was obtained after AT1 antagonism, however, when both SMC migration and proliferation were inhibited.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAUMGARTNER H. R. EINE NEUE METHODE ZUR ERZEUGUNG VON THROMBEN DURCH GEZIELTE UBERDEHNUNG DER GEFAESSWAND. Z Gesamte Exp Med. 1963 Sep 12;137:227–247. [PubMed] [Google Scholar]
- Bell L., Madri J. A. Influence of the angiotensin system on endothelial and smooth muscle cell migration. Am J Pathol. 1990 Jul;137(1):7–12. [PMC free article] [PubMed] [Google Scholar]
- Bumpus F. M., Catt K. J., Chiu A. T., DeGasparo M., Goodfriend T., Husain A., Peach M. J., Taylor D. G., Jr, Timmermans P. B. Nomenclature for angiotensin receptors. A report of the Nomenclature Committee of the Council for High Blood Pressure Research. Hypertension. 1991 May;17(5):720–721. doi: 10.1161/01.hyp.17.5.720. [DOI] [PubMed] [Google Scholar]
- Bunkenburg B., Schnell C., Baum H. P., Cumin F., Wood J. M. Prolonged angiotensin II antagonism in spontaneously hypertensive rats. Hemodynamic and biochemical consequences. Hypertension. 1991 Sep;18(3):278–288. doi: 10.1161/01.hyp.18.3.278. [DOI] [PubMed] [Google Scholar]
- Chiu A. T., Roscoe W. A., McCall D. E., Timmermans P. B. Angiotensin II-1 receptors mediate both vasoconstrictor and hypertrophic responses in rat aortic smooth muscle cells. Receptor. 1991;1(3):133–140. [PubMed] [Google Scholar]
- Clowes A. W., Reidy M. A., Clowes M. M. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983 Sep;49(3):327–333. [PubMed] [Google Scholar]
- Clowes A. W., Reidy M. A., Clowes M. M. Mechanisms of stenosis after arterial injury. Lab Invest. 1983 Aug;49(2):208–215. [PubMed] [Google Scholar]
- Clowes A. W., Schwartz S. M. Significance of quiescent smooth muscle migration in the injured rat carotid artery. Circ Res. 1985 Jan;56(1):139–145. doi: 10.1161/01.res.56.1.139. [DOI] [PubMed] [Google Scholar]
- Daemen M. J., Lombardi D. M., Bosman F. T., Schwartz S. M. Angiotensin II induces smooth muscle cell proliferation in the normal and injured rat arterial wall. Circ Res. 1991 Feb;68(2):450–456. doi: 10.1161/01.res.68.2.450. [DOI] [PubMed] [Google Scholar]
- Dudley D. T., Panek R. L., Major T. C., Lu G. H., Bruns R. F., Klinkefus B. A., Hodges J. C., Weishaar R. E. Subclasses of angiotensin II binding sites and their functional significance. Mol Pharmacol. 1990 Sep;38(3):370–377. [PubMed] [Google Scholar]
- Ferns G. A., Raines E. W., Sprugel K. H., Motani A. S., Reidy M. A., Ross R. Inhibition of neointimal smooth muscle accumulation after angioplasty by an antibody to PDGF. Science. 1991 Sep 6;253(5024):1129–1132. doi: 10.1126/science.1653454. [DOI] [PubMed] [Google Scholar]
- Fingerle J., Johnson R., Clowes A. W., Majesky M. W., Reidy M. A. Role of platelets in smooth muscle cell proliferation and migration after vascular injury in rat carotid artery. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8412–8416. doi: 10.1073/pnas.86.21.8412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg I. D., Stemerman M. B., Schnipper L. E., Ransil B. J., Crooks G. W., Fuhro R. L. Vascular smooth muscle cell kinetics: a new assay for studying patterns of cellular proliferation in vivo. Science. 1979 Aug 31;205(4409):920–922. doi: 10.1126/science.472713. [DOI] [PubMed] [Google Scholar]
- Hutchison A. J., Webb R. L., Oei H. H., Ghai G. R., Zimmerman M. B., Williams M. CGS 21680C, an A2 selective adenosine receptor agonist with preferential hypotensive activity. J Pharmacol Exp Ther. 1989 Oct;251(1):47–55. [PubMed] [Google Scholar]
- Murphy T. J., Alexander R. W., Griendling K. K., Runge M. S., Bernstein K. E. Isolation of a cDNA encoding the vascular type-1 angiotensin II receptor. Nature. 1991 May 16;351(6323):233–236. doi: 10.1038/351233a0. [DOI] [PubMed] [Google Scholar]
- Okunishi H., Miyazaki M., Okamura T., Toda N. Different distribution of two types of angiotensin II-generating enzymes in the aortic wall. Biochem Biophys Res Commun. 1987 Dec 31;149(3):1186–1192. doi: 10.1016/0006-291x(87)90533-x. [DOI] [PubMed] [Google Scholar]
- Powell J. S., Clozel J. P., Müller R. K., Kuhn H., Hefti F., Hosang M., Baumgartner H. R. Inhibitors of angiotensin-converting enzyme prevent myointimal proliferation after vascular injury. Science. 1989 Jul 14;245(4914):186–188. doi: 10.1126/science.2526370. [DOI] [PubMed] [Google Scholar]
- Powell J. S., Müller R. K., Rouge M., Kuhn H., Hefti F., Baumgartner H. R. The proliferative response to vascular injury is suppressed by angiotensin-converting enzyme inhibition. J Cardiovasc Pharmacol. 1990;16 (Suppl 4):S42–S49. doi: 10.1097/00005344-199016004-00010. [DOI] [PubMed] [Google Scholar]
- Rosenthal J. H., Pfeifle B., Michailov M. L., Pschorr J., Jacob I. C., Dahlheim H. Investigations of components of the renin-angiotensin system in rat vascular tissue. Hypertension. 1984 May-Jun;6(3):383–390. doi: 10.1161/01.hyp.6.3.383. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Stemerman M. B., Benditt E. P. The aortic intima. II. Repair of the aortic lining after mechanical denudation. Am J Pathol. 1975 Oct;81(1):15–42. [PMC free article] [PubMed] [Google Scholar]
- Spaet T. H., Stemerman M. B., Veith F. J., Lejnieks I. Intimal injury and regrowth in the rabbit aorta; medial smooth muscle cells as a source of neointima. Circ Res. 1975 Jan;36(1):58–70. doi: 10.1161/01.res.36.1.58. [DOI] [PubMed] [Google Scholar]
- Wong P. C., Hart S. D., Zaspel A. M., Chiu A. T., Ardecky R. J., Smith R. D., Timmermans P. B. Functional studies of nonpeptide angiotensin II receptor subtype-specific ligands: DuP 753 (AII-1) and PD123177 (AII-2). J Pharmacol Exp Ther. 1990 Nov;255(2):584–592. [PubMed] [Google Scholar]


