Abstract
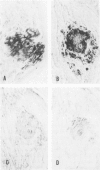
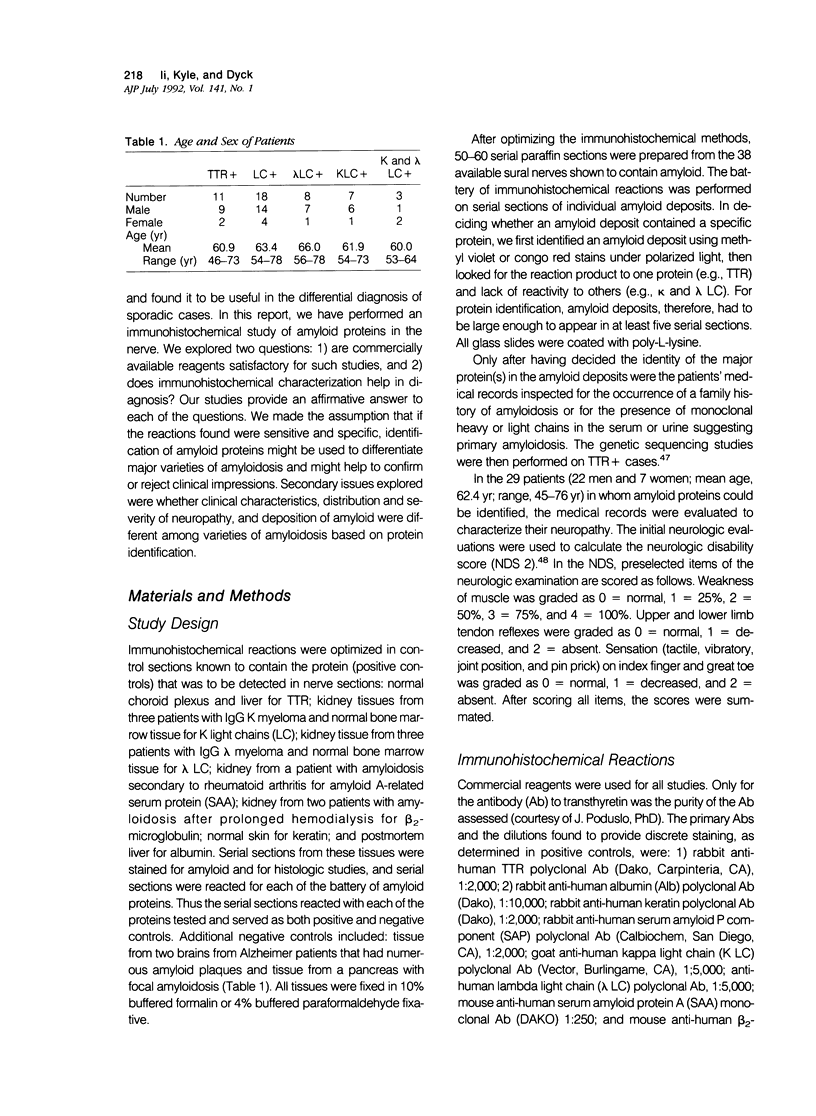
To test whether immunohistochemical characterization of proteins in amyloid deposits in biopsied sural nerves gives reliable and useful diagnostic information using commercially available reagents, biopsy specimens of sural nerves from 38 patients with amyloid neuropathy were studied. Transthyretin (TTR) was detected in the amyloid deposits of 11 nerves, lambda light chains (LC) in 8 nerves, kappa LC in 7 nerves, and both lambda and kappa LC in 3 nerves. In 9 nerves, the amyloid deposits were too small to allow adequate immunohistochemical characterization of amyloid proteins in serial sections. Evidence that immunohistochemical characterization was correct came from: 1) evaluation of kin, 2) search for monoclonal proteins in the plasma, and 3) sequencing of the gene abnormalities in TTR+ cases. In 9 of 11 TTR+ cases, in which DNA could be obtained, sequencing of the gene showed that each of the 9 cases was heterozygous for a gene mutation; 7 had previously described mutations and 2 undescribed mutations. Therefore, in the nine sporadic cases without plasma monoclonal light chains, the immunohistochemical characterization correctly identified the protein in amyloid as transthyretin. Likewise, there was a high concordance between immunoglobulin light chains in plasma and light chains in amyloid in primary amyloidosis. Evaluation of the type, distribution, and severity of the neurologic symptoms and deficits showed: 1) the sensorimotor and autonomic neuropathy of amyloidosis characteristically affects proximal as well as distal limbs, and 2) the type of amyloidosis probably cannot be determined from the characteristics or severity of the neuropathy alone or from the location or size of amyloid deposits in nerve.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki S., Mawatari S., Ohta M., Nakajima A., Kuroiwa Y. Polyneuritic amyloidosis in a Japanese family. Arch Neurol. 1968 Jun;18(6):593–602. doi: 10.1001/archneur.1968.00470360015001. [DOI] [PubMed] [Google Scholar]
- Breathnach S. M., Bhogal B., Dyck R. F., De Beer F. C., Black M. M., Pepys M. B. Immunohistochemical demonstration of amyloid P component in skin of normal subjects and patients with cutaneous amyloidosis. Br J Dermatol. 1981 Aug;105(2):115–124. doi: 10.1111/j.1365-2133.1981.tb01195.x. [DOI] [PubMed] [Google Scholar]
- Coria F., Castaño E., Prelli F., Larrondo-Lillo M., van Duinen S., Shelanski M. L., Frangione B. Isolation and characterization of amyloid P component from Alzheimer's disease and other types of cerebral amyloidosis. Lab Invest. 1988 Apr;58(4):454–458. [PubMed] [Google Scholar]
- Costa P. P., Figueira A. S., Bravo F. R. Amyloid fibril protein related to prealbumin in familial amyloidotic polyneuropathy. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4499–4503. doi: 10.1073/pnas.75.9.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalakas M. C., Cunningham G. Characterization of amyloid deposits in biopsies of 15 with "sporadic" (non-familial or plasma cell dyscrasia amyloid polyneuropathy. Acta Neuropathol. 1986;69(1-2):66–72. doi: 10.1007/BF00687040. [DOI] [PubMed] [Google Scholar]
- Dwulet F. E., Benson M. D. Characterization of a transthyretin (prealbumin) variant associated with familial amyloidotic polyneuropathy type II (Indiana/Swiss). J Clin Invest. 1986 Oct;78(4):880–886. doi: 10.1172/JCI112675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwulet F. E., Benson M. D. Polymorphism of human plasma thyroxine binding prealbumin. Biochem Biophys Res Commun. 1983 Jul 29;114(2):657–662. doi: 10.1016/0006-291x(83)90831-8. [DOI] [PubMed] [Google Scholar]
- Dyck P. J., Lambert E. H. Dissociated sensation in amylidosis. Compound action potential, quantitative histologic and teased-fiber, and electron microscopic studies of sural nerve biopsies. Arch Neurol. 1969 May;20(5):490–507. doi: 10.1001/archneur.1969.00480110054005. [DOI] [PubMed] [Google Scholar]
- Dyck P. J., Sherman W. R., Hallcher L. M., Service F. J., O'Brien P. C., Grina L. A., Palumbo P. J., Swanson C. J. Human diabetic endoneurial sorbitol, fructose, and myo-inositol related to sural nerve morphometry. Ann Neurol. 1980 Dec;8(6):590–596. doi: 10.1002/ana.410080608. [DOI] [PubMed] [Google Scholar]
- Feurle G. E., Linke R. P., Kuhn E., Wagner A. Clinical value of immunohistochemistry with AF-antibody in the diagnosis of familial amyloid neuropathy. J Neurol. 1984;231(5):237–243. doi: 10.1007/BF00313658. [DOI] [PubMed] [Google Scholar]
- Fujihara S. Differentiation of amyloid fibril proteins in tissue sections. Two simple and reliable histological methods applied to fifty-one cases of systemic amyloidosis. Acta Pathol Jpn. 1982 Sep;32(5):771–782. doi: 10.1111/j.1440-1827.1982.tb03191.x. [DOI] [PubMed] [Google Scholar]
- Gertz M. A., Kyle R. A. Primary systemic amyloidosis--a diagnostic primer. Mayo Clin Proc. 1989 Dec;64(12):1505–1519. doi: 10.1016/s0025-6196(12)65706-1. [DOI] [PubMed] [Google Scholar]
- Glenner G. G. Amyloid deposits and amyloidosis. The beta-fibrilloses (first of two parts). N Engl J Med. 1980 Jun 5;302(23):1283–1292. doi: 10.1056/NEJM198006053022305. [DOI] [PubMed] [Google Scholar]
- Glenner G. G. Amyloid deposits and amyloidosis: the beta-fibrilloses (second of two parts). N Engl J Med. 1980 Jun 12;302(24):1333–1343. doi: 10.1056/NEJM198006123022403. [DOI] [PubMed] [Google Scholar]
- Goffin Y. A. Amyloid proteins and amyloidoses: complexity updated. Acta Clin Belg. 1989;44(1):37–51. doi: 10.1080/17843286.1989.11717984. [DOI] [PubMed] [Google Scholar]
- Gorevic P. D., Prelli F. C., Wright J., Pras M., Frangione B. Systemic senile amyloidosis. Identification of a new prealbumin (transthyretin) variant in cardiac tissue: immunologic and biochemical similarity to one form of familial amyloidotic polyneuropathy. J Clin Invest. 1989 Mar;83(3):836–843. doi: 10.1172/JCI113966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorevic P. D., Prelli F. C., Wright J., Pras M., Frangione B. Systemic senile amyloidosis. Identification of a new prealbumin (transthyretin) variant in cardiac tissue: immunologic and biochemical similarity to one form of familial amyloidotic polyneuropathy. J Clin Invest. 1989 Mar;83(3):836–843. doi: 10.1172/JCI113966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson S., Proper J. A., Bowie E. J., Sommer S. S. Parameters affecting the yield of DNA from human blood. Anal Biochem. 1987 Sep;165(2):294–299. doi: 10.1016/0003-2697(87)90272-7. [DOI] [PubMed] [Google Scholar]
- Haltia M., Prelli F., Ghiso J., Kiuru S., Somer H., Palo J., Frangione B. Amyloid protein in familial amyloidosis (Finnish type) is homologous to gelsolin, an actin-binding protein. Biochem Biophys Res Commun. 1990 Mar 30;167(3):927–932. doi: 10.1016/0006-291x(90)90612-q. [DOI] [PubMed] [Google Scholar]
- Harada T., Kito S., Shimoyama M., Katayama S., Sasaki H., Furuya H., Yoshioka K., Sakaki Y. Genetic and clinical studies of Japanese patients with familial amyloid polyneuropathy. Eur Neurol. 1989;29(1):48–52. doi: 10.1159/000116392. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Hui A. N., Koss M. N., Hochholzer L., Wehunt W. D. Amyloidosis presenting in the lower respiratory tract. Clinicopathologic, radiologic, immunohistochemical, and histochemical studies on 48 cases. Arch Pathol Lab Med. 1986 Mar;110(3):212–218. [PubMed] [Google Scholar]
- Husby G., Ranløv P. J., Sletten K., Marhaug G. The amyloid in familial amyloid cardiomyopathy of Danish origin is related to pre-albumin. Clin Exp Immunol. 1985 Apr;60(1):207–216. [PMC free article] [PubMed] [Google Scholar]
- Ii K., Hizawa K., Nunomura S., Morizumi H. Systemic amyloid myopathy--light-microscopic and fine-structural study of the skeletal muscles with histochemical and immunohistochemical study of amyloid. Acta Neuropathol. 1984;64(2):114–121. doi: 10.1007/BF00695574. [DOI] [PubMed] [Google Scholar]
- Ii S., Minnerath S., Ii K., Dyck P. J., Sommer S. S. Two-tiered DNA-based diagnosis of transthyretin amyloidosis reveals two novel point mutations. Neurology. 1991 Jun;41(6):893–898. doi: 10.1212/wnl.41.6.893. [DOI] [PubMed] [Google Scholar]
- Ii S., Minnerath S., Ii K., Dyck P. J., Sommer S. S. Two-tiered DNA-based diagnosis of transthyretin amyloidosis reveals two novel point mutations. Neurology. 1991 Jun;41(6):893–898. doi: 10.1212/wnl.41.6.893. [DOI] [PubMed] [Google Scholar]
- Jacobson D. R., Gorevic P. D., Buxbaum J. N. A homozygous transthyretin variant associated with senile systemic amyloidosis: evidence for a late-onset disease of genetic etiology. Am J Hum Genet. 1990 Jul;47(1):127–136. [PMC free article] [PubMed] [Google Scholar]
- Jacobson D. R., Santiago-Schwartz F., Buxbaum J. N. Restriction fragment analysis confirms the position 33 mutation in transthyretin from an Israeli patient (SKO) with familial amyloidotic polyneuropathy. Biochem Biophys Res Commun. 1988 May 31;153(1):198–202. doi: 10.1016/s0006-291x(88)81208-7. [DOI] [PubMed] [Google Scholar]
- Jones L. A., Skare J. C., Harding J. A., Cohen A. S., Milunsky A., Skinner M. Proline at position 36: a new transthyretin mutation associated with familial amyloidotic polyneuropathy. Am J Hum Genet. 1991 May;48(5):979–982. [PMC free article] [PubMed] [Google Scholar]
- Kametani F., Tonoike H., Hoshi A., Shinoda T., Kito S. A variant prealbumin-related low molecular weight amyloid fibril protein in familial amyloid polyneuropathy of Japanese origin. Biochem Biophys Res Commun. 1984 Dec 14;125(2):622–628. doi: 10.1016/0006-291x(84)90584-9. [DOI] [PubMed] [Google Scholar]
- Kedar I., Sohar E., Ravid M. Degradation of amyloid by a serum component and inhibition of degradation. J Lab Clin Med. 1982 May;99(5):693–700. [PubMed] [Google Scholar]
- Kitamoto T., Tashima T., Tateishi J. Novel histochemical approaches to the prealbumin-related senile and familial forms of systemic amyloidosis. Am J Pathol. 1986 Jun;123(3):407–412. [PMC free article] [PubMed] [Google Scholar]
- Koeppen A. H., Mitzen E. J., Hans M. B., Peng S. K., Bailey R. O. Familial amyloid polyneuropathy. Muscle Nerve. 1985 Nov-Dec;8(9):733–749. doi: 10.1002/mus.880080902. [DOI] [PubMed] [Google Scholar]
- Kyle R. A., Bayrd E. D. Amyloidosis: review of 236 cases. Medicine (Baltimore) 1975 Jul;54(4):271–299. doi: 10.1097/00005792-197507000-00001. [DOI] [PubMed] [Google Scholar]
- Löfberg H., Grubb A. O., Nilsson E. K., Jensson O., Gudmundsson G., Blöndal H., Arnason A., Thorsteinsson L. Immunohistochemical characterization of the amyloid deposits and quantitation of pertinent cerebrospinal fluid proteins in hereditary cerebral hemorrhage with amyloidosis. Stroke. 1987 Mar-Apr;18(2):431–440. doi: 10.1161/01.str.18.2.431. [DOI] [PubMed] [Google Scholar]
- Maury C. P., Teppo A. M., Karinemi A. L., Koeppen A. H. Amyloid fibril protein in familial amyloidosis with cranial neuropathy and corneal lattice dystrophy (FAP type IV) is related to transthyretin. Am J Clin Pathol. 1988 Mar;89(3):359–364. doi: 10.1093/ajcp/89.3.359. [DOI] [PubMed] [Google Scholar]
- Nakazato M., Kangawa K., Minamino N., Tawara S., Matsuo H., Araki S. Revised analysis of amino acid replacement in a prealbumin variant (SKO-III) associated with familial amyloidotic polyneuropathy of Jewish origin. Biochem Biophys Res Commun. 1984 Sep 28;123(3):921–928. doi: 10.1016/s0006-291x(84)80222-3. [DOI] [PubMed] [Google Scholar]
- Nichols W. C., Gregg R. E., Brewer H. B., Jr, Benson M. D. A mutation in apolipoprotein A-I in the Iowa type of familial amyloidotic polyneuropathy. Genomics. 1990 Oct;8(2):318–323. doi: 10.1016/0888-7543(90)90288-6. [DOI] [PubMed] [Google Scholar]
- Nichols W. C., Liepnieks J. J., McKusick V. A., Benson M. D. Direct sequencing of the gene for Maryland/German familial amyloidotic polyneuropathy type II and genotyping by allele-specific enzymatic amplification. Genomics. 1989 Oct;5(3):535–540. doi: 10.1016/0888-7543(89)90020-7. [DOI] [PubMed] [Google Scholar]
- Nordlie M., Sletten K., Husby G., Ranløv P. J. A new prealbumin variant in familial amyloid cardiomyopathy of Danish origin. Scand J Immunol. 1988 Jan;27(1):119–122. doi: 10.1111/j.1365-3083.1988.tb02329.x. [DOI] [PubMed] [Google Scholar]
- Picken M. M., Pelton K., Frangione B., Gallo G. Primary amyloidosis A. Immunohistochemical and biochemical characterization. Am J Pathol. 1987 Dec;129(3):536–542. [PMC free article] [PubMed] [Google Scholar]
- Poduslo J. F., Curran G. L., Dyck P. J. Increase in albumin, IgG, and IgM blood-nerve barrier indices in human diabetic neuropathy. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4879–4883. doi: 10.1073/pnas.85.13.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pras M., Prelli F., Franklin E. C., Frangione B. Primary structure of an amyloid prealbumin variant in familial polyneuropathy of Jewish origin. Proc Natl Acad Sci U S A. 1983 Jan;80(2):539–542. doi: 10.1073/pnas.80.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva M. J., Almeida M. R., Alves I. L., Moreira P., Gawinowicz M., Costa P. P., Rauh S., Banhzoff A., Altland K. Molecular analyses of an acidic transthyretin Asn 90 variant. Am J Hum Genet. 1991 May;48(5):1004–1008. [PMC free article] [PubMed] [Google Scholar]
- Saraiva M. J., Birken S., Costa P. P., Goodman D. S. Amyloid fibril protein in familial amyloidotic polyneuropathy, Portuguese type. Definition of molecular abnormality in transthyretin (prealbumin). J Clin Invest. 1984 Jul;74(1):104–119. doi: 10.1172/JCI111390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skare J. C., Saraiva M. J., Alves I. L., Skare I. B., Milunsky A., Cohen A. S., Skinner M. A new mutation causing familial amyloidotic polyneuropathy. Biochem Biophys Res Commun. 1989 Nov 15;164(3):1240–1246. doi: 10.1016/0006-291x(89)91802-0. [DOI] [PubMed] [Google Scholar]
- Stein K., Störkel S., Linke R. P., Goebel H. H. Chemical heterogeneity of amyloid in the carpal tunnel syndrome. Virchows Arch A Pathol Anat Histopathol. 1987;412(1):37–45. doi: 10.1007/BF00750729. [DOI] [PubMed] [Google Scholar]
- Stoflet E. S., Koeberl D. D., Sarkar G., Sommer S. S. Genomic amplification with transcript sequencing. Science. 1988 Jan 29;239(4839):491–494. doi: 10.1126/science.3340835. [DOI] [PubMed] [Google Scholar]
- Tawara S., Araki S., Toshimori K., Nakagawa H., Ohtaki S. Amyloid fibril protein in type I familial amyloidotic polyneuropathy in Japanese. J Lab Clin Med. 1981 Dec;98(6):811–822. [PubMed] [Google Scholar]
- Tawara S., Nakazato M., Kangawa K., Matsuo H., Araki S. Identification of amyloid prealbumin variant in familial amyloidotic polyneuropathy (Japanese type). Biochem Biophys Res Commun. 1983 Nov 15;116(3):880–888. doi: 10.1016/s0006-291x(83)80224-1. [DOI] [PubMed] [Google Scholar]
- Thomas P. K., King R. H. Peripheral nerve changes in amyloid neuropathy. Brain. 1974 Jun;97(2):395–406. doi: 10.1093/brain/97.1.395. [DOI] [PubMed] [Google Scholar]
- Ueno S., Uemichi T., Yorifuji S., Tarui S. A novel variant of transthyretin (Tyr114 to Cys) deduced from the nucleotide sequences of gene fragments from familial amyloidotic polyneuropathy in Japanese sibling cases. Biochem Biophys Res Commun. 1990 May 31;169(1):143–147. doi: 10.1016/0006-291x(90)91445-x. [DOI] [PubMed] [Google Scholar]
- Vinters H. V., Pardridge W. M., Secor D. L., Ishii N. Immunohistochemical study of cerebral amyloid angiopathy. II. Enhancement of immunostaining using formic acid pretreatment of tissue sections. Am J Pathol. 1988 Oct;133(1):150–162. [PMC free article] [PubMed] [Google Scholar]
- Vinters H. V., Pardridge W. M., Yang J. Immunohistochemical study of cerebral amyloid angiopathy: use of an antiserum to a synthetic 28-amino-acid peptide fragment of the Alzheimer's disease amyloid precursor. Hum Pathol. 1988 Feb;19(2):214–222. doi: 10.1016/s0046-8177(88)80352-6. [DOI] [PubMed] [Google Scholar]
- Wallace M. R., Conneally P. M., Benson M. D. A DNA test for Indiana/Swiss hereditary amyloidosis (FAP II). Am J Hum Genet. 1988 Aug;43(2):182–187. [PMC free article] [PubMed] [Google Scholar]
- Wallace M. R., Dwulet F. E., Conneally P. M., Benson M. D. Biochemical and molecular genetic characterization of a new variant prealbumin associated with hereditary amyloidosis. J Clin Invest. 1986 Jul;78(1):6–12. doi: 10.1172/JCI112573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermark P., Shirahama T., Skinner M., Brun A., Cameron R., Cohen A. S. Immunohistochemical evidence for the lack of amyloid P component in some intracerebral amyloids. Lab Invest. 1982 May;46(5):457–460. [PubMed] [Google Scholar]
- Westermark P., Sletten K., Johansson B., Cornwell G. G., 3rd Fibril in senile systemic amyloidosis is derived from normal transthyretin. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2843–2845. doi: 10.1073/pnas.87.7.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M., Tsukagoshi H., Satoh J., Ishiai S., Nakazato M., Furuya H., Sasaki H., Sakaki Y., Yokota T. "Sporadic" prealbumin-related amyloid polyneuropathy: report of two cases. J Neurol. 1987 Dec;235(2):69–73. doi: 10.1007/BF00718012. [DOI] [PubMed] [Google Scholar]
- Zimmerman I. R., Karnes J. L., O'Brien P. C., Dyck P. J. Imaging system for nerve and fiber tract morphometry: components, approaches, performance, and results. J Neuropathol Exp Neurol. 1980 Jul;39(4):409–419. doi: 10.1097/00005072-198007000-00002. [DOI] [PubMed] [Google Scholar]