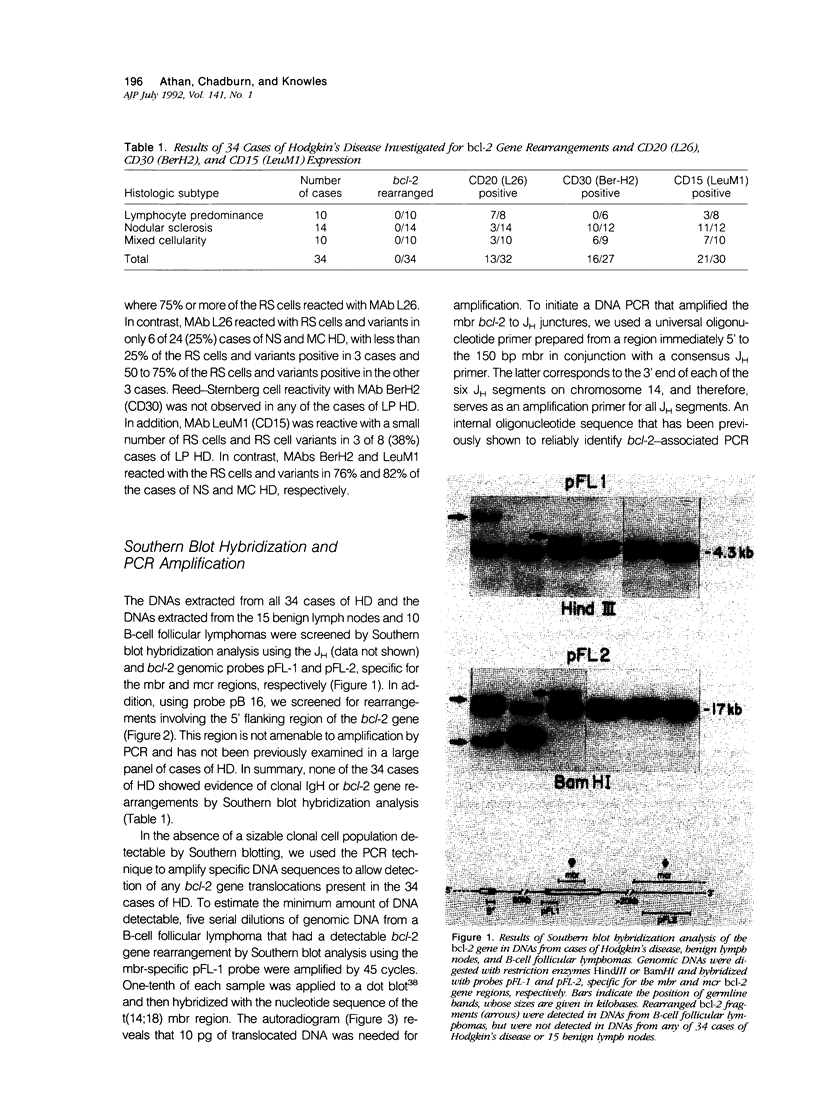
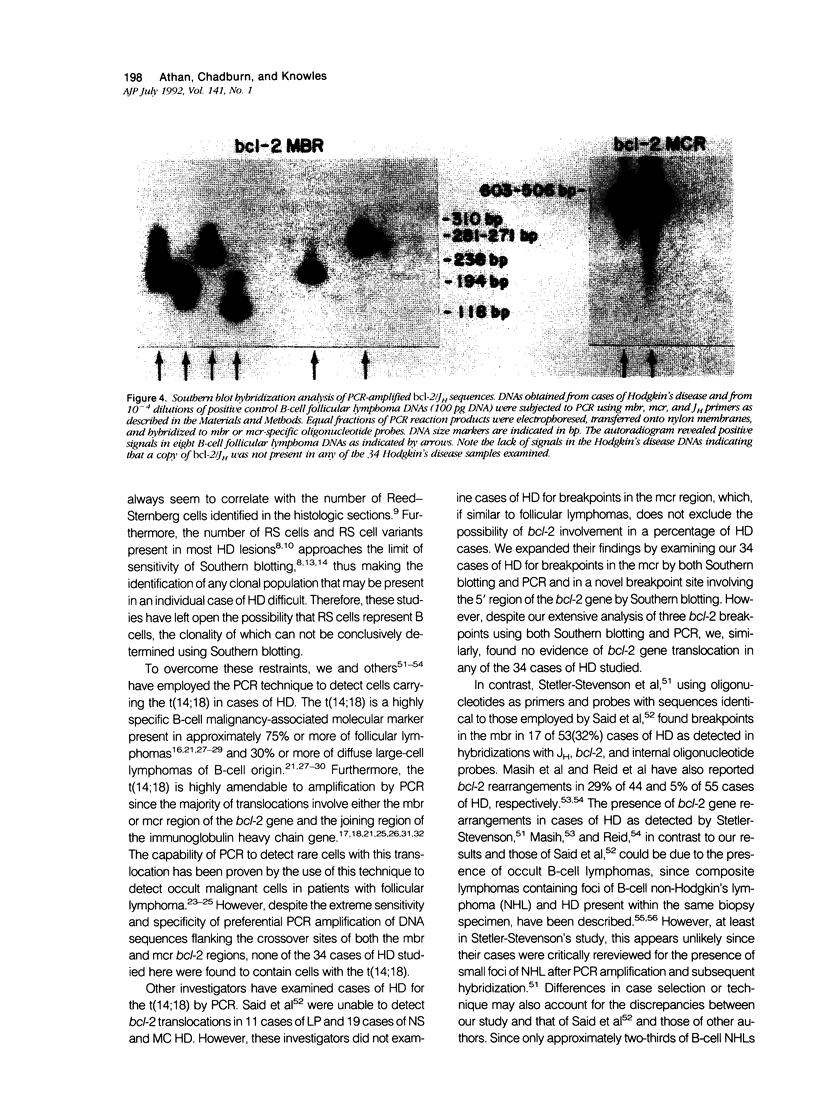
Abstract
B-cell associated antigens are frequently expressed by the Reed-Sternberg (RS) cells of lymphocyte predominance (LP) Hodgkin's disease (HD) and are sometimes expressed by those of nodular sclerosis (NS) and mixed cellularity (MC) HD. Clonal immunoglobulin gene rearrangements have been detected in some HD cases as well. These findings suggest that at least some cases of HD may be of B-cell derivation. Rearrangements of the bcl-2 gene, associated with the t(14;18)(q32;q21) are present in more than 75% of follicular and 30% of diffuse lymphomas of B-cell origin, suggesting that this translocation plays an important role in B-cell lymphomagenesis. In this study, we investigated 34 cases of HD (10 LP, 14 NS, and 10 MC) for bcl-2 gene rearrangements to determine if this B-cell lymphoma-associated translocation also plays a role in the pathogenesis of HD. The cases of HD were analyzed by Southern blot hybridization, using DNA probes that detect the major and minor breakpoint cluster regions and a 5'bcl-2 breakpoint region recently cloned and found to be involved in B-cell chronic lymphocytic leukemia, and by the polymerase chain reaction (PCR), using oligonucleotides capable of amplifying and detecting the major breakpoint region (mbr) and minor cluster region (mcr) breakpoint regions in t(14;18). bcl-2 translocations were not detected in any of the 34 cases of HD by Southern blot hybridization or by PCR. This is in spite of the fact that RS cells expressing B-cell-associated antigen CD20 were detectable in 7/8 cases of LP HD and 6/24 cases of NS and MC HD with monoclonal antibody L26. Therefore, these results indicate that the bcl-2 gene translocation does not play an important role in the pathogenesis of HD and did not provide evidence for the B-cell origin of HD.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi M., Cossman J., Longo D., Croce C. M., Tsujimoto Y. Variant translocation of the bcl-2 gene to immunoglobulin lambda light chain gene in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2771–2774. doi: 10.1073/pnas.86.8.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnarsson B. A., Kadin M. E. The immunophenotype of Reed-Sternberg cells. A study of 50 cases of Hodgkin's disease using fixed frozen tissues. Cancer. 1989 Jun 1;63(11):2083–2087. doi: 10.1002/1097-0142(19890601)63:11<2083::aid-cncr2820631102>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Aisenberg A. C., Wilkes B. M., Jacobson J. O. The bcl-2 gene is rearranged in many diffuse B-cell lymphomas. Blood. 1988 Apr;71(4):969–972. [PubMed] [Google Scholar]
- Bakhshi A., Jensen J. P., Goldman P., Wright J. J., McBride O. W., Epstein A. L., Korsmeyer S. J. Cloning the chromosomal breakpoint of t(14;18) human lymphomas: clustering around JH on chromosome 14 and near a transcriptional unit on 18. Cell. 1985 Jul;41(3):899–906. doi: 10.1016/s0092-8674(85)80070-2. [DOI] [PubMed] [Google Scholar]
- Bakhshi A., Wright J. J., Graninger W., Seto M., Owens J., Cossman J., Jensen J. P., Goldman P., Korsmeyer S. J. Mechanism of the t(14;18) chromosomal translocation: structural analysis of both derivative 14 and 18 reciprocal partners. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2396–2400. doi: 10.1073/pnas.84.8.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinker M. G., Poppema S., Buys C. H., Timens W., Osinga J., Visser L. Clonal immunoglobulin gene rearrangements in tissues involved by Hodgkin's disease. Blood. 1987 Jul;70(1):186–191. [PubMed] [Google Scholar]
- Cartun R. W., Coles F. B., Pastuszak W. T. Utilization of monoclonal antibody L26 in the identification and confirmation of B-cell lymphomas. A sensitive and specific marker applicable to formalin-and B5-fixed, paraffin-embedded tissues. Am J Pathol. 1987 Dec;129(3):415–421. [PMC free article] [PubMed] [Google Scholar]
- Casey T. T., Olson S. J., Cousar J. B., Collins R. D. Immunophenotypes of Reed-Sternberg cells: a study of 19 cases of Hodgkin's disease in plastic-embedded sections. Blood. 1989 Dec;74(8):2624–2628. [PubMed] [Google Scholar]
- Chadburn A., Athan E., Wieczorek R., Knowles D. M. Detection and characterization of human T-cell lymphotropic virus type I (HTLV-I) associated T-cell neoplasms in an HTLV-I nonendemic region by polymerase chain reaction. Blood. 1991 Jun 1;77(11):2419–2430. [PubMed] [Google Scholar]
- Chadburn A., Inghirami G., Knowles D. M. T-cell activation-associated antigen expression by neoplastic T-cells. Hematol Pathol. 1992;6(3):131–141. [PubMed] [Google Scholar]
- Cleary M. L., Chao J., Warnke R., Sklar J. Immunoglobulin gene rearrangement as a diagnostic criterion of B-cell lymphoma. Proc Natl Acad Sci U S A. 1984 Jan;81(2):593–597. doi: 10.1073/pnas.81.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary M. L., Galili N., Sklar J. Detection of a second t(14;18) breakpoint cluster region in human follicular lymphomas. J Exp Med. 1986 Jul 1;164(1):315–320. doi: 10.1084/jem.164.1.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary M. L., Sklar J. Nucleotide sequence of a t(14;18) chromosomal breakpoint in follicular lymphoma and demonstration of a breakpoint-cluster region near a transcriptionally active locus on chromosome 18. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7439–7443. doi: 10.1073/pnas.82.21.7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crescenzi M., Seto M., Herzig G. P., Weiss P. D., Griffith R. C., Korsmeyer S. J. Thermostable DNA polymerase chain amplification of t(14;18) chromosome breakpoints and detection of minimal residual disease. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4869–4873. doi: 10.1073/pnas.85.13.4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr C. J., Saiki R. K., Erlich H. A., McCormick F., Marshall C. J. Analysis of RAS gene mutations in acute myeloid leukemia by polymerase chain reaction and oligonucleotide probes. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1629–1633. doi: 10.1073/pnas.85.5.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gonzalez C. L., Medeiros L. J., Jaffe E. S. Composite lymphoma. A clinicopathologic analysis of nine patients with Hodgkin's disease and B-cell non-Hodgkin's lymphoma. Am J Clin Pathol. 1991 Jul;96(1):81–89. doi: 10.1093/ajcp/96.1.81. [DOI] [PubMed] [Google Scholar]
- Inghirami G., Zhu B. Y., Chess L., Knowles D. M. Flow cytometric and immunohistochemical characterization of the gamma/delta T-lymphocyte population in normal human lymphoid tissue and peripheral blood. Am J Pathol. 1990 Feb;136(2):357–367. [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. S. The elusive Reed-Sternberg cell. N Engl J Med. 1989 Feb 23;320(8):529–531. doi: 10.1056/NEJM198902233200813. [DOI] [PubMed] [Google Scholar]
- Jones D. B. The histogenesis of the Reed-Sternberg cell and its mononuclear counterparts. J Pathol. 1987 Mar;151(3):191–195. doi: 10.1002/path.1711510306. [DOI] [PubMed] [Google Scholar]
- Kadin M. E. Histogenesis of Hodgkin's disease: possible insights from a comparison with lymphomatoid papulosis. Hum Pathol. 1987 Nov;18(11):1085–1088. doi: 10.1016/s0046-8177(87)80373-8. [DOI] [PubMed] [Google Scholar]
- Kadin M. E., Muramoto L., Said J. Expression of T-cell antigens on Reed-Sternberg cells in a subset of patients with nodular sclerosing and mixed cellularity Hodgkin's disease. Am J Pathol. 1988 Feb;130(2):345–353. [PMC free article] [PubMed] [Google Scholar]
- Kim H., Hendrickson R., Dorfman R. F. Composite lymphoma. Cancer. 1977 Sep;40(3):959–976. doi: 10.1002/1097-0142(197709)40:3<959::aid-cncr2820400302>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Knowles D. M., 2nd, Neri A., Pelicci P. G., Burke J. S., Wu A., Winberg C. D., Sheibani K., Dalla-Favera R. Immunoglobulin and T-cell receptor beta-chain gene rearrangement analysis of Hodgkin's disease: implications for lineage determination and differential diagnosis. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7942–7946. doi: 10.1073/pnas.83.20.7942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles D. M., Athan E., Ubriaco A., McNally L., Inghirami G., Wieczorek R., Finfer M., Jakobiec F. A. Extranodal noncutaneous lymphoid hyperplasias represent a continuous spectrum of B-cell neoplasia: demonstration by molecular genetic analysis. Blood. 1989 May 1;73(6):1635–1645. [PubMed] [Google Scholar]
- Korsmeyer S. J., Hieter P. A., Ravetch J. V., Poplack D. G., Waldmann T. A., Leder P. Developmental hierarchy of immunoglobulin gene rearrangements in human leukemic pre-B-cells. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7096–7100. doi: 10.1073/pnas.78.11.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. S., Blick M. B., Pathak S., Trujillo J. M., Butler J. J., Katz R. L., McLaughlin P., Hagemeister F. B., Velasquez W. S., Goodacre A. The gene located at chromosome 18 band q21 is rearranged in uncultured diffuse lymphomas as well as follicular lymphomas. Blood. 1987 Jul;70(1):90–95. [PubMed] [Google Scholar]
- Lee M. S., Chang K. S., Cabanillas F., Freireich E. J., Trujillo J. M., Stass S. A. Detection of minimal residual cells carrying the t(14;18) by DNA sequence amplification. Science. 1987 Jul 10;237(4811):175–178. doi: 10.1126/science.3110950. [DOI] [PubMed] [Google Scholar]
- Lipford E., Wright J. J., Urba W., Whang-Peng J., Kirsch I. R., Raffeld M., Cossman J., Longo D. L., Bakhshi A., Korsmeyer S. J. Refinement of lymphoma cytogenetics by the chromosome 18q21 major breakpoint region. Blood. 1987 Dec;70(6):1816–1823. [PubMed] [Google Scholar]
- Mason D. Y., Comans-Bitter W. M., Cordell J. L., Verhoeven M. A., van Dongen J. J. Antibody L26 recognizes an intracellular epitope on the B-cell-associated CD20 antigen. Am J Pathol. 1990 Jun;136(6):1215–1222. [PMC free article] [PubMed] [Google Scholar]
- Ngan B. Y., Nourse J., Cleary M. L. Detection of chromosomal translocation t(14;18) within the minor cluster region of bcl-2 by polymerase chain reaction and direct genomic sequencing of the enzymatically amplified DNA in follicular lymphomas. Blood. 1989 May 15;73(7):1759–1762. [PubMed] [Google Scholar]
- O'Connor N. T., Crick J. A., Gatter K. C., Mason D. Y., Falini B., Stein H. S. Cell lineage in Hodgkin's disease. Lancet. 1987 Jan 17;1(8525):158–158. doi: 10.1016/s0140-6736(87)91987-8. [DOI] [PubMed] [Google Scholar]
- Offit K., Koduru P. R., Hollis R., Filippa D., Jhanwar S. C., Clarkson B. C., Chaganti R. S. 18q21 rearrangement in diffuse large cell lymphoma: incidence and clinical significance. Br J Haematol. 1989 Jun;72(2):178–183. doi: 10.1111/j.1365-2141.1989.tb07680.x. [DOI] [PubMed] [Google Scholar]
- Pinkus G. S., Said J. W. Hodgkin's disease, lymphocyte predominance type, nodular--a distinct entity? Unique staining profile for L&H variants of Reed-Sternberg cells defined by monoclonal antibodies to leukocyte common antigen, granulocyte-specific antigen, and B-cell-specific antigen. Am J Pathol. 1985 Jan;118(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- Pinkus G. S., Said J. W. Hodgkin's disease, lymphocyte predominance type, nodular--further evidence for a B cell derivation. L & H variants of Reed-Sternberg cells express L26, a pan B cell marker. Am J Pathol. 1988 Nov;133(2):211–217. [PMC free article] [PubMed] [Google Scholar]
- Roth M. S., Schnitzer B., Bingham E. L., Harnden C. E., Hyder D. M., Ginsburg D. Rearrangement of immunoglobulin and T-cell receptor genes in Hodgkin's disease. Am J Pathol. 1988 May;131(2):331–338. [PMC free article] [PubMed] [Google Scholar]
- Said J. W., Sassoon A. F., Shintaku I. P., Kurtin P. J., Pinkus G. S. Absence of bcl-2 major breakpoint region and JH gene rearrangement in lymphocyte predominance Hodgkin's disease. Results of Southern blot analysis and polymerase chain reaction. Am J Pathol. 1991 Feb;138(2):261–264. [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stashenko P., Nadler L. M., Hardy R., Schlossman S. F. Characterization of a human B lymphocyte-specific antigen. J Immunol. 1980 Oct;125(4):1678–1685. [PubMed] [Google Scholar]
- Stein H., Hansmann M. L., Lennert K., Brandtzaeg P., Gatter K. C., Mason D. Y. Reed-Sternberg and Hodgkin cells in lymphocyte-predominant Hodgkin's disease of nodular subtype contain J chain. Am J Clin Pathol. 1986 Sep;86(3):292–297. doi: 10.1093/ajcp/86.3.292. [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson M., Crush-Stanton S., Cossman J. Involvement of the bcl-2 gene in Hodgkin's disease. J Natl Cancer Inst. 1990 May 16;82(10):855–858. doi: 10.1093/jnci/82.10.855. [DOI] [PubMed] [Google Scholar]
- Stetlet-Stevenson M., Raffeld M., Cohen P., Cossman J. Detection of occult follicular lymphoma by specific DNA amplification. Blood. 1988 Nov;72(5):1822–1825. [PubMed] [Google Scholar]
- Sundeen J., Lipford E., Uppenkamp M., Sussman E., Wahl L., Raffeld M., Cossman J. Rearranged antigen receptor genes in Hodgkin's disease. Blood. 1987 Jul;70(1):96–103. [PubMed] [Google Scholar]
- Tsujimoto Y., Croce C. M. Analysis of the structure, transcripts, and protein products of bcl-2, the gene involved in human follicular lymphoma. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5214–5218. doi: 10.1073/pnas.83.14.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto Y., Gorham J., Cossman J., Jaffe E., Croce C. M. The t(14;18) chromosome translocations involved in B-cell neoplasms result from mistakes in VDJ joining. Science. 1985 Sep 27;229(4720):1390–1393. doi: 10.1126/science.3929382. [DOI] [PubMed] [Google Scholar]
- Weiss L. M., Strickler J. G., Hu E., Warnke R. A., Sklar J. Immunoglobulin gene rearrangements in Hodgkin's disease. Hum Pathol. 1986 Oct;17(10):1009–1014. doi: 10.1016/s0046-8177(86)80084-3. [DOI] [PubMed] [Google Scholar]
- Weiss L. M., Warnke R. A., Sklar J., Cleary M. L. Molecular analysis of the t(14;18) chromosomal translocation in malignant lymphomas. N Engl J Med. 1987 Nov 5;317(19):1185–1189. doi: 10.1056/NEJM198711053171904. [DOI] [PubMed] [Google Scholar]
- Wieczorek R., Buck D., Bindl J., Knowles D. M. Monoclonal antibody Leu-22 (L60) permits the demonstration of some neoplastic T cells in routinely fixed and paraffin-embedded tissue sections. Hum Pathol. 1988 Dec;19(12):1434–1443. doi: 10.1016/s0046-8177(88)80236-3. [DOI] [PubMed] [Google Scholar]
- Yunis J. J., Oken M. M., Kaplan M. E., Ensrud K. M., Howe R. R., Theologides A. Distinctive chromosomal abnormalities in histologic subtypes of non-Hodgkin's lymphoma. N Engl J Med. 1982 Nov 11;307(20):1231–1236. doi: 10.1056/NEJM198211113072002. [DOI] [PubMed] [Google Scholar]