Abstract
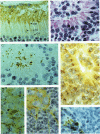
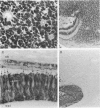
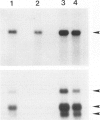
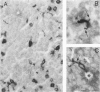
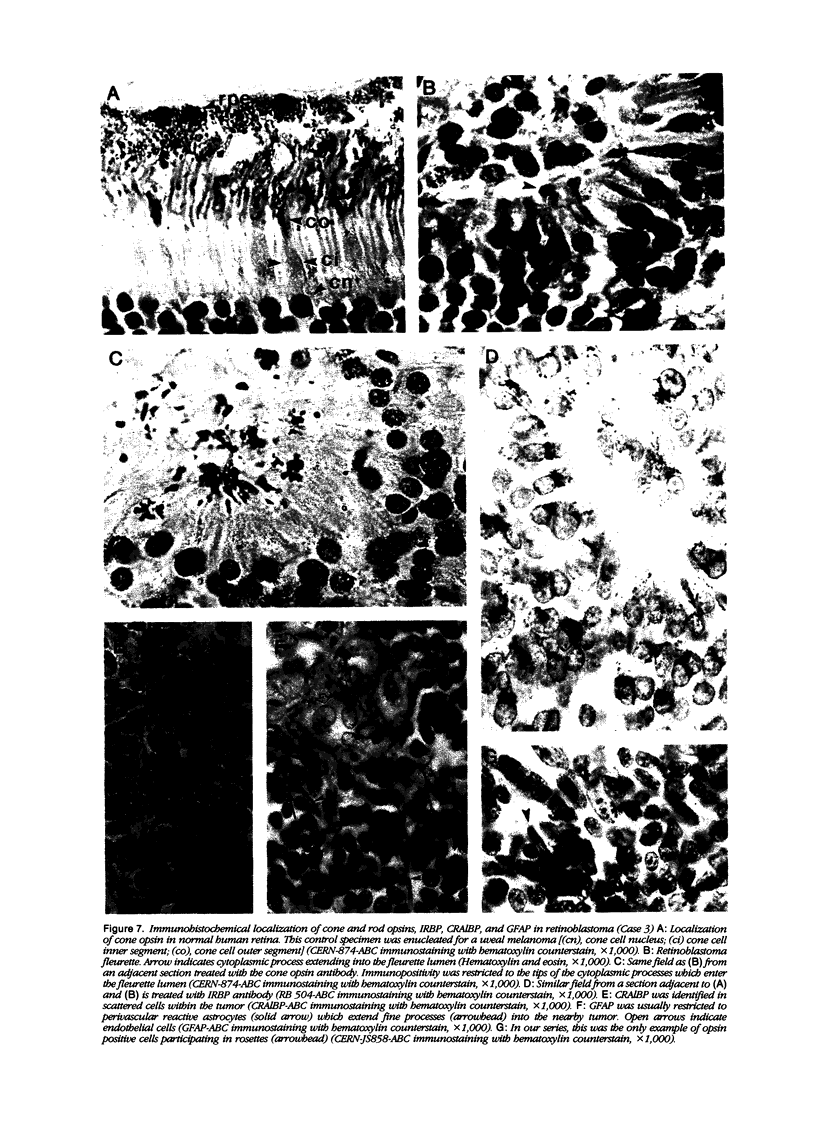
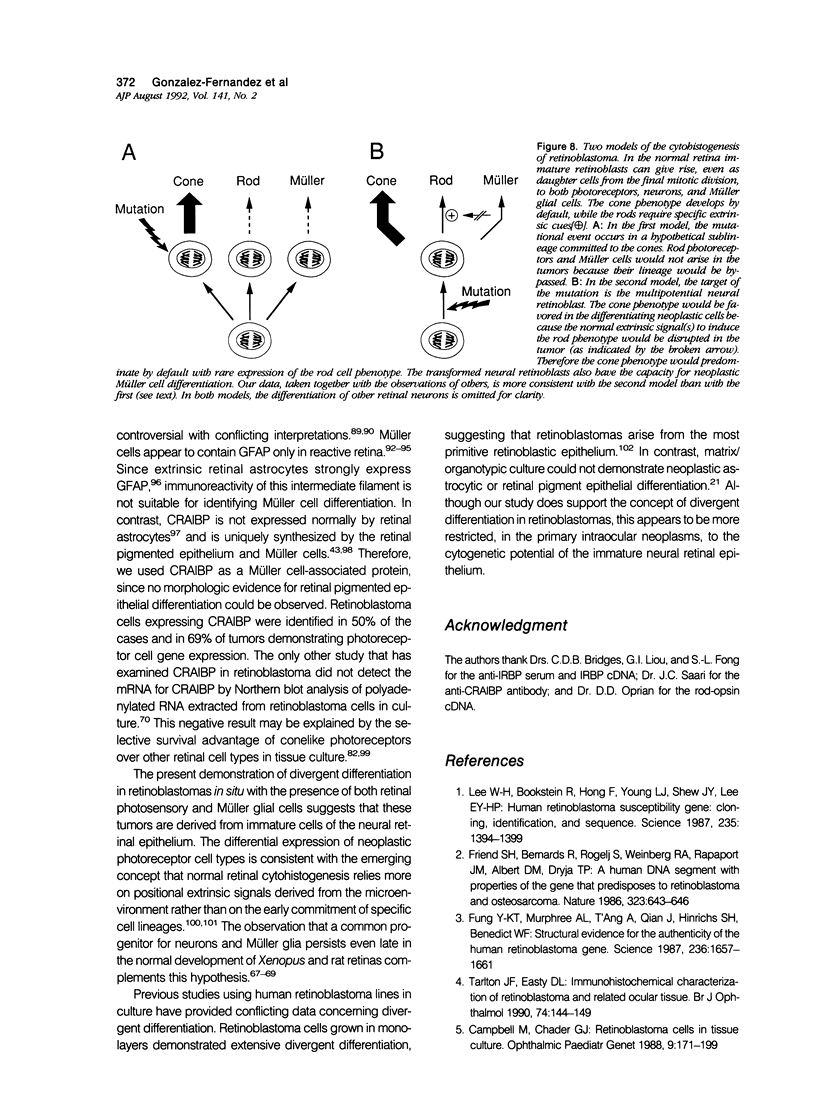
Retinoblastoma, the most common intraocular tumor of childhood, is a malignant neoplasm that arises during retinal development. The embryonal cell target for neoplastic transformation is not yet clearly defined. To better understand the histogenetic potential of this tumor, the expression of photoreceptor and glial cell-associated proteins were examined in 22 primary retinoblastomas. Interphotoreceptor retinol-binding protein (IRBP), cone and rod opsins were selected as the photoreceptor specific proteins due to their different temporal patterns of expression during normal retinal development. Neoplastic Müller cell differentiation, and non-neoplastic reactive astrocytes were identified using cellular retinaldehyde binding-protein (CRAlBP), and glial fibrillary acidic protein (GFAP), respectively. Photoreceptor proteins were present in 16 cases and showed different cellular patterns of expression. IRBP and cone opsin were usually abundant. Although rod opsin was clearly identified in eight tumors, its expression was more restricted than either IRBP or cone opsin. This differential pattern of expression, opposite to the normal pattern of photoreceptor gene expression in the adult retina, corresponded to a marked decrease in mRNA for rod opsin. Cone opsin and IRBP colocalized in fleurettes demonstrating that neoplastic human cone cells are capable of IRBP synthesis. Müller cell differentiation was present in 12 of the 16 cases in which photoreceptor proteins were detected. In contrast, GFAP was only present in reactive, stromal astrocytes associated with blood vessels. Our data suggest that the retinoblastoma has the histogenetic potential of the immature neural retinal epithelium which can give rise to both photoreceptor and Müller cell lineages. The differential expression of cone and rod phenotypes in retinoblastoma is consistent with the "default" mechanism of cone cell differentiation.
Full text
PDF
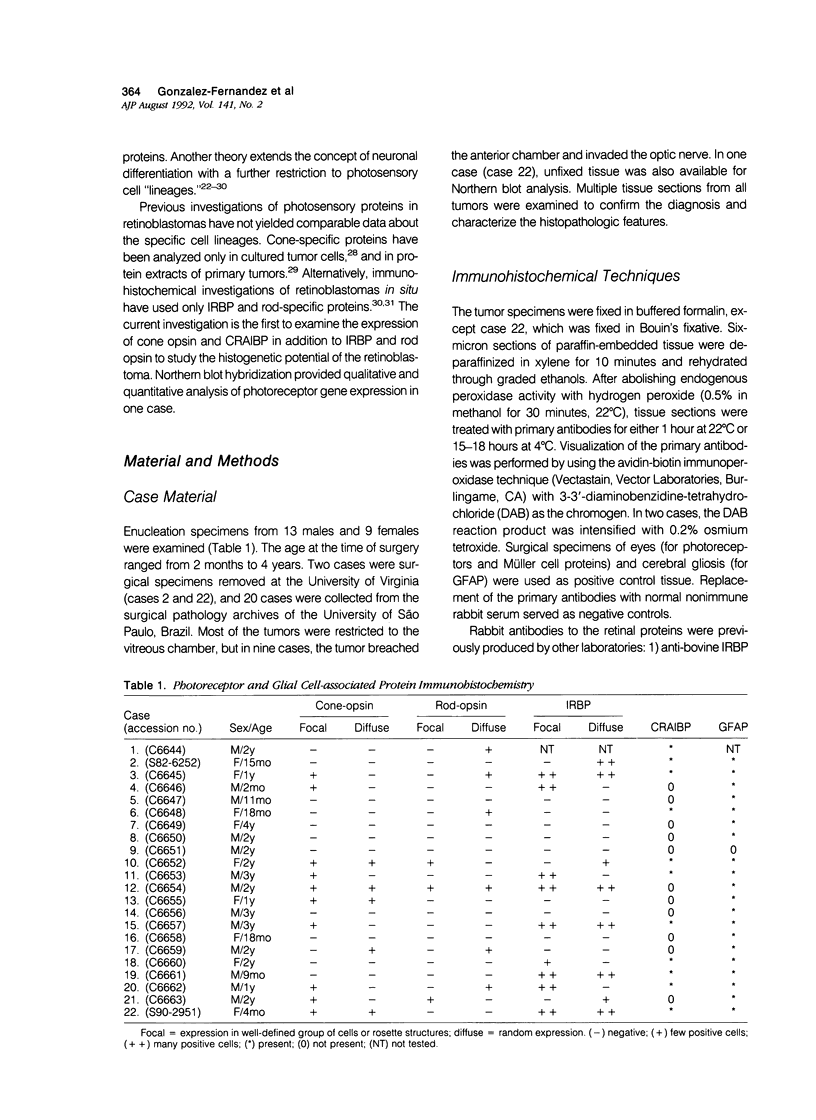


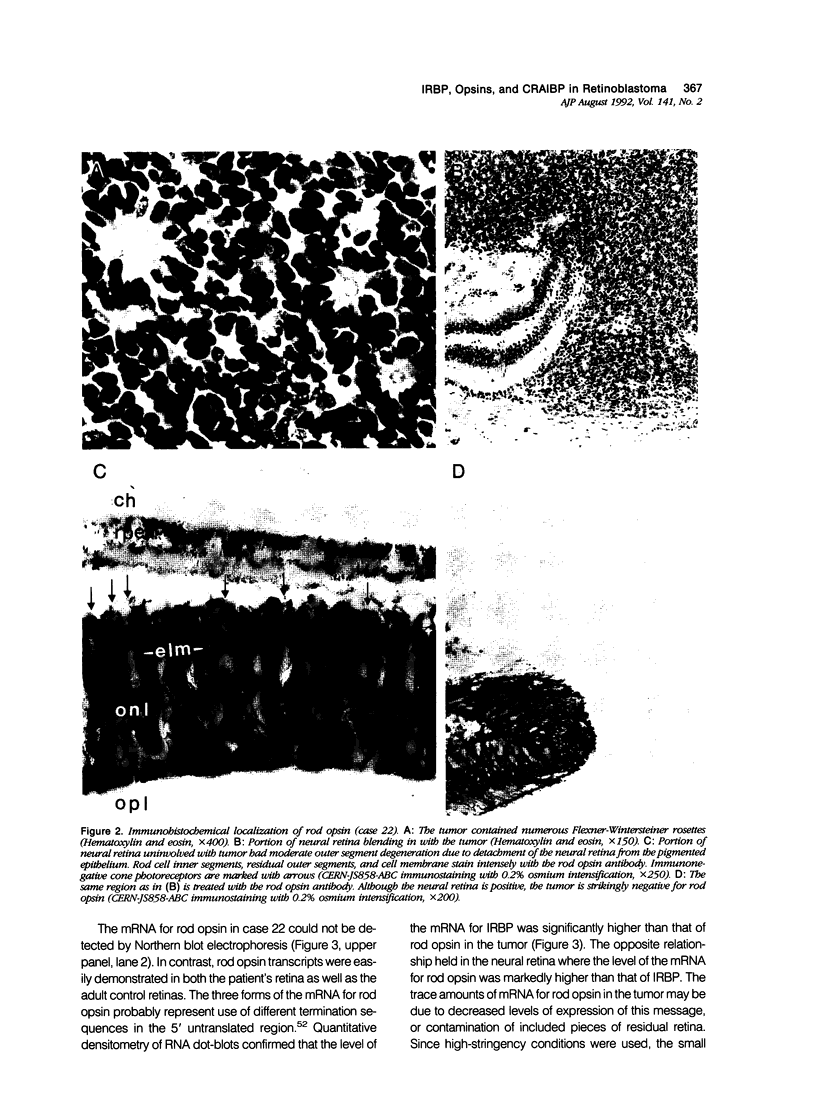
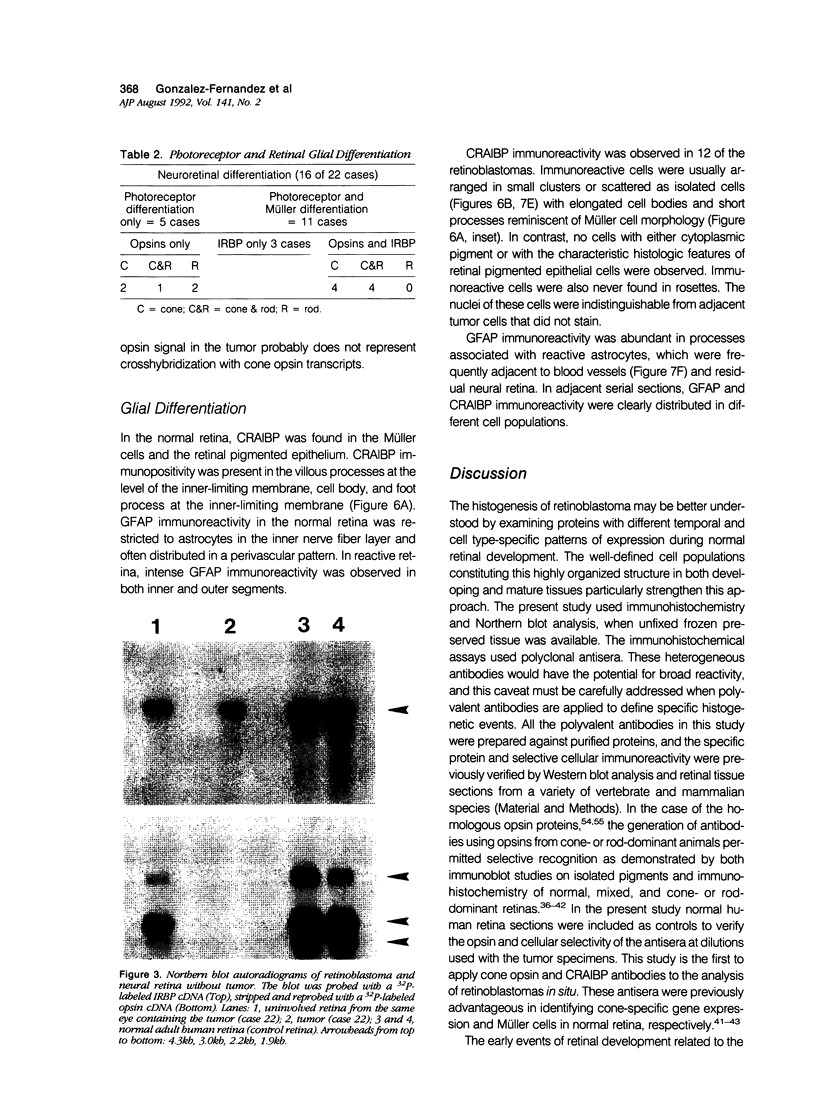
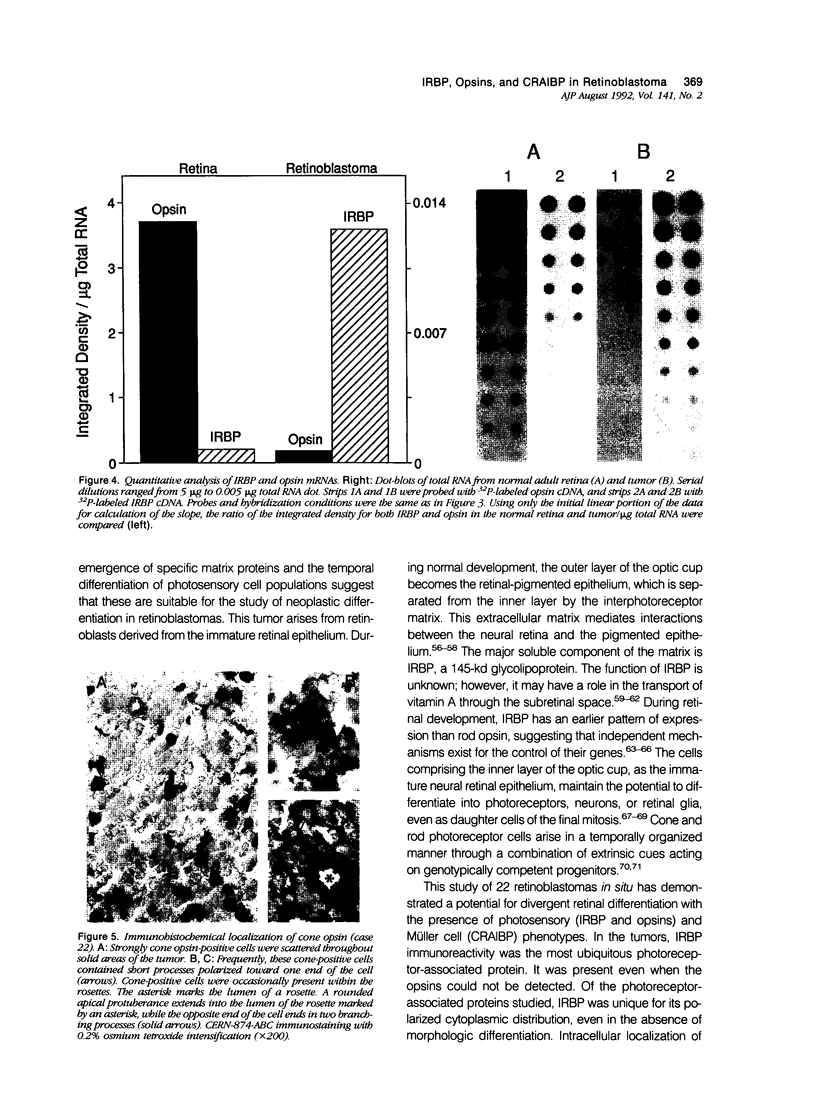




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler R., Hatlee M. Plasticity and differentiation of embryonic retinal cells after terminal mitosis. Science. 1989 Jan 20;243(4889):391–393. doi: 10.1126/science.2911751. [DOI] [PubMed] [Google Scholar]
- Adler R., Lindsey J. D., Elsner C. L. Expression of cone-like properties by chick embryo neural retina cells in glial-free monolayer cultures. J Cell Biol. 1984 Sep;99(3):1173–1178. doi: 10.1083/jcb.99.3.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applebury M. L., Hargrave P. A. Molecular biology of the visual pigments. Vision Res. 1986;26(12):1881–1895. doi: 10.1016/0042-6989(86)90115-x. [DOI] [PubMed] [Google Scholar]
- Baehr W., Falk J. D., Bugra K., Triantafyllos J. T., McGinnis J. F. Isolation and analysis of the mouse opsin gene. FEBS Lett. 1988 Oct 10;238(2):253–256. doi: 10.1016/0014-5793(88)80490-3. [DOI] [PubMed] [Google Scholar]
- Banerjee U., Zipursky S. L. The role of cell-cell interaction in the development of the Drosophila visual system. Neuron. 1990 Feb;4(2):177–187. doi: 10.1016/0896-6273(90)90093-u. [DOI] [PubMed] [Google Scholar]
- Bentley P. J., Grubb B. R. Lateral spatial assay of zinc and superoxide dismutase in the ocular lens. Invest Ophthalmol Vis Sci. 1990 Jan;31(1):153–155. [PubMed] [Google Scholar]
- Bignami A., Dahl D. The radial glia of Müller in the rat retina and their response to injury. An immunofluorescence study with antibodies to the glial fibrillary acidic (GFA) protein. Exp Eye Res. 1979 Jan;28(1):63–69. doi: 10.1016/0014-4835(79)90106-4. [DOI] [PubMed] [Google Scholar]
- Björklund H., Dahl D. Glial fibrillary acidic protein (GFAP)-like immunoreactivity in the rodent eye. Comparison between peripheral glia of the anterior uvea and central glia of the retina. J Neuroimmunol. 1985 Jun;8(4-6):331–345. doi: 10.1016/s0165-5728(85)80071-0. [DOI] [PubMed] [Google Scholar]
- Bogenmann E., Lochrie M. A., Simon M. I. Cone cell-specific genes expressed in retinoblastoma. Science. 1988 Apr 1;240(4848):76–78. doi: 10.1126/science.2451289. [DOI] [PubMed] [Google Scholar]
- Bunt-Milam A. H., Saari J. C. Immunocytochemical localization of two retinoid-binding proteins in vertebrate retina. J Cell Biol. 1983 Sep;97(3):703–712. doi: 10.1083/jcb.97.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M. A., Karras P., Chader G. J. Y-79 retinoblastoma cells: isolation and characterization of clonal lineages. Exp Eye Res. 1989 Jan;48(1):77–85. doi: 10.1016/0014-4835(89)90021-3. [DOI] [PubMed] [Google Scholar]
- Campbell M., Chader G. J. Retinoblastoma cells in tissue culture. Ophthalmic Paediatr Genet. 1988 Nov;9(3):171–199. doi: 10.3109/13816818809031495. [DOI] [PubMed] [Google Scholar]
- Carter-Dawson L., Alvarez R. A., Fong S. L., Liou G. I., Sperling H. G., Bridges C. D. Rhodopsin, 11-cis vitamin A, and interstitial retinol-binding protein (IRBP) during retinal development in normal and rd mutant mice. Dev Biol. 1986 Aug;116(2):431–438. doi: 10.1016/0012-1606(86)90144-2. [DOI] [PubMed] [Google Scholar]
- Chader G. J. Interphotoreceptor retinoid-binding protein (IRBP): a model protein for molecular biological and clinically relevant studies. Friedenwald lecture. Invest Ophthalmol Vis Sci. 1989 Jan;30(1):7–22. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Choux R., Tripier M. F., Bérard M., Hassoun J., Toga M. Ultrastructure d'une tumeur de la rétine. Bull Cancer. 1972 Apr-Jun;59(3):301–314. [PubMed] [Google Scholar]
- Craft J. L., Sang D. N., Dryja T. P., Brockhurst R. J., Robinson N. L., Albert D. M. Glial cell component in retinoblastoma. Exp Eye Res. 1985 May;40(5):647–659. doi: 10.1016/0014-4835(85)90134-4. [DOI] [PubMed] [Google Scholar]
- Detrick B., Chader G. J., Rodrigues M., Kyritsis A. P., Chan C. C., Hooks J. J. Coexpression of neuronal, glial, and major histocompatibility complex class II antigens on retinoblastoma cells. Cancer Res. 1988 Mar 15;48(6):1633–1641. [PubMed] [Google Scholar]
- Dickson D. H., Ramsey M. S., Tonus J. G. Synapse formation in retinoblastoma tumours. Br J Ophthalmol. 1976 May;60(5):371–375. doi: 10.1136/bjo.60.5.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoso L. A., Shields C. L., Lee E. Y. Immunohistochemistry of retinoblastoma. A review. Ophthalmic Paediatr Genet. 1989 Mar;10(1):3–32. doi: 10.3109/13816818909083770. [DOI] [PubMed] [Google Scholar]
- Eisenfeld A. J., Bunt-Milam A. H., Saari J. C. Immunocytochemical localization of interphotoreceptor retinoid-binding protein in developing normal and RCS rat retinas. Invest Ophthalmol Vis Sci. 1985 May;26(5):775–778. [PubMed] [Google Scholar]
- Eisenfeld A. J., Bunt-Milam A. H., Sarthy P. V. Müller cell expression of glial fibrillary acidic protein after genetic and experimental photoreceptor degeneration in the rat retina. Invest Ophthalmol Vis Sci. 1984 Nov;25(11):1321–1328. [PubMed] [Google Scholar]
- Fager L. Y., Fager R. S. Chromatographic separation of rod and cone pigments from chicken retinas. Methods Enzymol. 1982;81:160–166. doi: 10.1016/s0076-6879(82)81027-6. [DOI] [PubMed] [Google Scholar]
- Feeney L. The interphotoreceptor space. I. Postnatal ontogeny in mice and rats. Dev Biol. 1973 May;32(1):101–114. doi: 10.1016/0012-1606(73)90223-6. [DOI] [PubMed] [Google Scholar]
- Fong S. L., Balakier H., Canton M., Bridges C. D., Gallie B. Retinoid-binding proteins in retinoblastoma tumors. Cancer Res. 1988 Mar 1;48(5):1124–1128. [PubMed] [Google Scholar]
- Fong S. L., Liou G. I., Landers R. A., Alvarez R. A., Bridges C. D. Purification and characterization of a retinol-binding glycoprotein synthesized and secreted by bovine neural retina. J Biol Chem. 1984 May 25;259(10):6534–6542. [PubMed] [Google Scholar]
- Fong S. L., Liou G. I., Landers R. A., Alvarez R. A., Gonzalez-Fernandez F., Glazebrook P. A., Lam D. M., Bridges C. D. Characterization, localization, and biosynthesis of an interstitial retinol-binding glycoprotein in the human eye. J Neurochem. 1984 Jun;42(6):1667–1676. doi: 10.1111/j.1471-4159.1984.tb12758.x. [DOI] [PubMed] [Google Scholar]
- Foster R. G., Korf H. W., Schalken J. J. Immunocytochemical markers revealing retinal and pineal but not hypothalamic photoreceptor systems in the Japanese quail. Cell Tissue Res. 1987 Apr;248(1):161–167. doi: 10.1007/BF01239977. [DOI] [PubMed] [Google Scholar]
- Foster R. G., Provencio I., Hudson D., Fiske S., De Grip W., Menaker M. Circadian photoreception in the retinally degenerate mouse (rd/rd). J Comp Physiol A. 1991 Jul;169(1):39–50. doi: 10.1007/BF00198171. [DOI] [PubMed] [Google Scholar]
- Friend S. H., Bernards R., Rogelj S., Weinberg R. A., Rapaport J. M., Albert D. M., Dryja T. P. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986 Oct 16;323(6089):643–646. doi: 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
- Fung Y. K., Murphree A. L., T'Ang A., Qian J., Hinrichs S. H., Benedict W. F. Structural evidence for the authenticity of the human retinoblastoma gene. Science. 1987 Jun 26;236(4809):1657–1661. doi: 10.1126/science.2885916. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Fernandez F., Healy J. I. Early expression of the gene for interphotoreceptor retinol-binding protein during photoreceptor differentiation suggests a critical role for the interphotoreceptor matrix in retinal development. J Cell Biol. 1990 Dec;111(6 Pt 1):2775–2784. doi: 10.1083/jcb.111.6.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Fernandez F., Landers R. A., Glazebrook P. A., Fong S. L., Liou G. I., Lam D. M., Bridges C. D. An extracellular retinol-binding glycoprotein in the eyes of mutant rats with retinal dystrophy: development, localization, and biosynthesis. J Cell Biol. 1984 Dec;99(6):2092–2098. doi: 10.1083/jcb.99.6.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman M. M., Perentes E., Katsetos C. D., Darcel F., Frankfurter A., Collins V. P., Donoso L. A., Eng L. F., Marangos P. J., Wiechmann A. F. Neuroblastic differentiation potential of the human retinoblastoma cell lines Y-79 and WERI-Rb1 maintained in an organ culture system. An immunohistochemical, electron microscopic, and biochemical study. Am J Pathol. 1989 Jan;134(1):115–132. [PMC free article] [PubMed] [Google Scholar]
- Hollyfield J. G., Fliesler S. J., Rayborn M. E., Fong S. L., Landers R. A., Bridges C. D. Synthesis and secretion of interstitial retinol-binding protein by the human retina. Invest Ophthalmol Vis Sci. 1985 Jan;26(1):58–67. [PubMed] [Google Scholar]
- Holt C. E., Bertsch T. W., Ellis H. M., Harris W. A. Cellular determination in the Xenopus retina is independent of lineage and birth date. Neuron. 1988 Mar;1(1):15–26. doi: 10.1016/0896-6273(88)90205-x. [DOI] [PubMed] [Google Scholar]
- Hurwitz R. L., Bogenmann E., Font R. L., Holcombe V., Clark D. Expression of the functional cone phototransduction cascade in retinoblastoma. J Clin Invest. 1990 Jun;85(6):1872–1878. doi: 10.1172/JCI114648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q., Lim R., Blodi F. C. Dual properties of cultured retinoblastoma cells: immunohistochemical characterization of neuronal and glial markers. Exp Eye Res. 1984 Aug;39(2):207–215. doi: 10.1016/0014-4835(84)90009-5. [DOI] [PubMed] [Google Scholar]
- Katsetos C. D., Herman M. M., Frankfurter A., Uffer S., Perentes E., Rubinstein L. J. Neuron-associated class III beta-tubulin isotype, microtubule-associated protein 2, and synaptophysin in human retinoblastomas in situ. Further immunohistochemical observations on the Flexner-Wintersteiner rosettes. Lab Invest. 1991 Jan;64(1):45–54. [PubMed] [Google Scholar]
- Kivelä T., Virtanen I., Marcus D. M., O'Brien J. M., Carpenter J. L., Brauner E., Tarkkanen A., Albert D. M. Neuronal and glial properties of a murine transgenic retinoblastoma model. Am J Pathol. 1991 May;138(5):1135–1148. [PMC free article] [PubMed] [Google Scholar]
- Kyritsis A. P., Fletcher R. T., Chader G. J. Laminin: an initiating role in retinoblastoma cell attachment and differentiation. In Vitro Cell Dev Biol. 1986 Jul;22(7):418–422. doi: 10.1007/BF02623532. [DOI] [PubMed] [Google Scholar]
- Kyritsis A. P., Tsokos M., Chader G. J. Control of retinoblastoma cell growth by differentiating agents: current work and future directions. Anticancer Res. 1986 May-Jun;6(3 Pt B):465–473. [PubMed] [Google Scholar]
- Kyritsis A. P., Tsokos M., Triche T. J., Chader G. J. Retinoblastoma: a primitive tumor with multipotential characteristics. Invest Ophthalmol Vis Sci. 1986 Dec;27(12):1760–1764. [PubMed] [Google Scholar]
- LaVail M. M., Pinto L. H., Yasumura D. The interphotoreceptor matrix in rats with inherited retinal dystrophy. Invest Ophthalmol Vis Sci. 1981 Nov;21(5):658–668. [PubMed] [Google Scholar]
- Lee W. H., Bookstein R., Hong F., Young L. J., Shew J. Y., Lee E. Y. Human retinoblastoma susceptibility gene: cloning, identification, and sequence. Science. 1987 Mar 13;235(4794):1394–1399. doi: 10.1126/science.3823889. [DOI] [PubMed] [Google Scholar]
- Lewis G. P., Erickson P. A., Kaska D. D., Fisher S. K. An immunocytochemical comparison of Müller cells and astrocytes in the cat retina. Exp Eye Res. 1988 Dec;47(6):839–853. doi: 10.1016/0014-4835(88)90067-x. [DOI] [PubMed] [Google Scholar]
- Margo C., Hidayat A., Kopelman J., Zimmerman L. E. Retinocytoma. A benign variant of retinoblastoma. Arch Ophthalmol. 1983 Oct;101(10):1519–1531. doi: 10.1001/archopht.1983.01040020521003. [DOI] [PubMed] [Google Scholar]
- Margry R. J., Jacobs C. W., Bonting S. L., De Grip W. J., Daemen F. J. Detergent-induced specificity of an antirhodopsin serum for opsin. Micro-complement fixation studies. Biochim Biophys Acta. 1983 Feb 15;742(3):463–470. doi: 10.1016/0167-4838(83)90262-5. [DOI] [PubMed] [Google Scholar]
- Matsumoto H., Yoshizawa T. Preparation of chicken iodopsin. Methods Enzymol. 1982;81:154–160. doi: 10.1016/s0076-6879(82)81026-4. [DOI] [PubMed] [Google Scholar]
- Messmer E. P., Font R. L., Kirkpatrick J. B., Höpping W. Immunohistochemical demonstration of neuronal and astrocytic differentiation in retinoblastoma. Ophthalmology. 1985 Jan;92(1):167–173. doi: 10.1016/s0161-6420(85)34076-9. [DOI] [PubMed] [Google Scholar]
- Miller N. M., Oberdorfer M. Neuronal and neuroglial responses following retinal lesions in the neonatal rats. J Comp Neurol. 1981 Nov 10;202(4):493–504. doi: 10.1002/cne.902020404. [DOI] [PubMed] [Google Scholar]
- Molnar M. L., Stefansson K., Marton L. S., Tripathi R. S., Molnar G. K. Immunohistochemistry of retinoblastomas in humans. Am J Ophthalmol. 1984 Mar;97(3):301–307. doi: 10.1016/0002-9394(84)90627-5. [DOI] [PubMed] [Google Scholar]
- Nathans J., Hogness D. S. Isolation, sequence analysis, and intron-exon arrangement of the gene encoding bovine rhodopsin. Cell. 1983 Oct;34(3):807–814. doi: 10.1016/0092-8674(83)90537-8. [DOI] [PubMed] [Google Scholar]
- Nathans J., Piantanida T. P., Eddy R. L., Shows T. B., Hogness D. S. Molecular genetics of inherited variation in human color vision. Science. 1986 Apr 11;232(4747):203–210. doi: 10.1126/science.3485310. [DOI] [PubMed] [Google Scholar]
- Nickerson J. M., Borst D. E., Redmond T. M., Si J. S., Toffenetti J., Chader G. J. The molecular biology of IRBP: application to problems of uveitis, protein chemistry, and evolution. Prog Clin Biol Res. 1991;362:139–161. [PubMed] [Google Scholar]
- Perentes E., Herbort C. P., Rubinstein L. J., Herman M. M., Uffer S., Donoso L. A., Collins V. P. Immunohistochemical characterization of human retinoblastomas in situ with multiple markers. Am J Ophthalmol. 1987 May 15;103(5):647–658. doi: 10.1016/s0002-9394(14)74324-7. [DOI] [PubMed] [Google Scholar]
- Politi L. E., Lee L., Wiggert B., Chader G., Adler R. Synthesis and secretion of interphotoreceptor retinoid-binding protein (IRBP) by isolated normal and rd mouse retinal photoreceptor neurons in culture. J Cell Physiol. 1989 Dec;141(3):682–690. doi: 10.1002/jcp.1041410329. [DOI] [PubMed] [Google Scholar]
- Popoff N. A., Ellsworth R. M. The fine structure of retinoblastoma. In vivo and in vitro observations. Lab Invest. 1971 Nov;25(5):389–402. [PubMed] [Google Scholar]
- Porrello K., Bhat S. P., Bok D. Detection of interphotoreceptor retinoid binding protein (IRBP) mRNA in human and cone-dominant squirrel retinas by in situ hybridization. J Histochem Cytochem. 1991 Feb;39(2):171–176. doi: 10.1177/39.2.1987260. [DOI] [PubMed] [Google Scholar]
- Rodrigues M. M., Hackett J., Gaskins R., Wiggert B., Lee L., Redmond M., Chader G. J. Interphotoreceptor retinoid-binding protein in retinal rod cells and pineal gland. Invest Ophthalmol Vis Sci. 1986 May;27(5):844–850. [PubMed] [Google Scholar]
- Rodrigues M. M., Wiggert B., Shields J., Donoso L., Bardenstein D., Katz N., Friendly D., Chader G. Retinoblastoma. Immunohistochemistry and cell differentiation. Ophthalmology. 1987 Apr;94(4):378–387. doi: 10.1016/s0161-6420(87)33448-7. [DOI] [PubMed] [Google Scholar]
- Rodrigues M. M., Wilson M. E., Wiggert B., Krishna G., Chader G. J. Retinoblastoma. A clinical, immunohistochemical, and electron microscopic case report. Ophthalmology. 1986 Aug;93(8):1010–1015. doi: 10.1016/s0161-6420(86)33630-3. [DOI] [PubMed] [Google Scholar]
- Rodrigues M., Hackett J., Wiggert B., Gery I., Spiegel A., Krishna G., Stein P., Chader G. Immunoelectron microscopic localization of photoreceptor-specific markers in the monkey retina. Curr Eye Res. 1987 Feb;6(2):369–380. doi: 10.3109/02713688709025190. [DOI] [PubMed] [Google Scholar]
- Saari J. C., Bredberg L., Garwin G. G. Identification of the endogenous retinoids associated with three cellular retinoid-binding proteins from bovine retina and retinal pigment epithelium. J Biol Chem. 1982 Nov 25;257(22):13329–13333. [PubMed] [Google Scholar]
- Schalken J. J., De Grip W. J. Enzyme-linked immunosorbent assay for quantitative determination of the visual pigment rhodopsin in total-eye extracts. Exp Eye Res. 1986 Sep;43(3):431–439. doi: 10.1016/s0014-4835(86)80078-1. [DOI] [PubMed] [Google Scholar]
- Schalken J. J., Margry R. J., De Grip W. J., Daemen F. J. A radioimmunoassay specific for opsin. Biochim Biophys Acta. 1983 Feb 15;742(3):471–476. doi: 10.1016/0167-4838(83)90263-7. [DOI] [PubMed] [Google Scholar]
- Schnitzer J. Immunocytochemical studies on the development of astrocytes, Müller (glial) cells, and oligodendrocytes in the rabbit retina. Brain Res Dev Brain Res. 1988 Nov 1;44(1):59–72. doi: 10.1016/0165-3806(88)90118-6. [DOI] [PubMed] [Google Scholar]
- Schrøder H. D. Immunohistochemical demonstration of glial markers in retinoblastomas. Virchows Arch A Pathol Anat Histopathol. 1987;411(1):67–72. doi: 10.1007/BF00734516. [DOI] [PubMed] [Google Scholar]
- Shaw G., Weber K. The structure and development of the rat retina: an immunofluorescence microscopical study using antibodies specific for intermediate filament proteins. Eur J Cell Biol. 1983 May;30(2):219–232. [PubMed] [Google Scholar]
- Stone J., Dreher Z. Relationship between astrocytes, ganglion cells and vasculature of the retina. J Comp Neurol. 1987 Jan 1;255(1):35–49. doi: 10.1002/cne.902550104. [DOI] [PubMed] [Google Scholar]
- Tarlton J. F., Easty D. L. Immunohistological characterisation of retinoblastoma and related ocular tissue. Br J Ophthalmol. 1990 Mar;74(3):144–149. doi: 10.1136/bjo.74.3.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor H. R., Carroll N., Jack I., Crock G. W. A scanning electron microscopic examination of retinoblastoma in tissue culture. Br J Ophthalmol. 1979 Aug;63(8):551–559. doi: 10.1136/bjo.63.8.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terenghi G., Polak J. M., Ballesta J., Cocchia D., Michetti F., Dahl D., Marangos P. J., Garner A. Immunocytochemistry of neuronal and glial markers in retinoblastoma. Virchows Arch A Pathol Anat Histopathol. 1984;404(1):61–73. doi: 10.1007/BF00704251. [DOI] [PubMed] [Google Scholar]
- Ts'o M. O., Fine B. S., Zimmerman L. E. The Flexner-Wintersteiner rosettes in retinoblastoma. Arch Pathol. 1969 Dec;88(6):664–671. [PubMed] [Google Scholar]
- Ts'o M. O., Fine B. S., Zimmerman L. E. The nature of retinoblastoma. II. Photoreceptor differentiation: an electron microscopic study. Am J Ophthalmol. 1970 Mar;69(3):350–359. doi: 10.1016/0002-9394(70)92264-6. [DOI] [PubMed] [Google Scholar]
- Ts'o M. O., Zimmerman L. E., Fine B. S. The nature of retinoblastoma. I. Photoreceptor differentiation: a clinical and histopathologic study. Am J Ophthalmol. 1970 Mar;69(3):339–349. doi: 10.1016/0002-9394(70)92263-4. [DOI] [PubMed] [Google Scholar]
- Tsokos M., Kyritsis A. P., Chader G. J., Triche T. J. Differentiation of human retinoblastoma in vitro into cell types with characteristics observed in embryonal or mature retina. Am J Pathol. 1986 Jun;123(3):542–552. [PMC free article] [PubMed] [Google Scholar]
- Turner D. L., Cepko C. L. A common progenitor for neurons and glia persists in rat retina late in development. Nature. 1987 Jul 9;328(6126):131–136. doi: 10.1038/328131a0. [DOI] [PubMed] [Google Scholar]
- Vrabec T., Arbizo V., Adamus G., McDowell J. H., Hargrave P. A., Donoso L. A. Rod cell-specific antigens in retinoblastoma. Arch Ophthalmol. 1989 Jul;107(7):1061–1063. doi: 10.1001/archopht.1989.01070020123044. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Raff M. C. Retinal astrocytes are immigrants from the optic nerve. Nature. 1988 Apr 28;332(6167):834–837. doi: 10.1038/332834a0. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Raff M. C. Rod photoreceptor development in vitro: intrinsic properties of proliferating neuroepithelial cells change as development proceeds in the rat retina. Neuron. 1990 Mar;4(3):461–467. doi: 10.1016/0896-6273(90)90058-n. [DOI] [PubMed] [Google Scholar]
- Wetts R., Fraser S. E. Multipotent precursors can give rise to all major cell types of the frog retina. Science. 1988 Mar 4;239(4844):1142–1145. doi: 10.1126/science.2449732. [DOI] [PubMed] [Google Scholar]
- Yen L., Fager R. S. Chromatographic resolution of the rod pigment from the four cone pigments of the chicken retina. Vision Res. 1984;24(11):1555–1562. doi: 10.1016/s0042-6989(84)80005-x. [DOI] [PubMed] [Google Scholar]
- ZIMMERMAN L. E., EASTHAM A. B. Acid mucopolysaccharide in the retinal pigment epithelium and visual cell layer of the developing mouse eye. Am J Ophthalmol. 1959 Jan;47(1 Pt 2):488–499. doi: 10.1016/s0002-9394(14)78054-7. [DOI] [PubMed] [Google Scholar]
- van Veen T., Katial A., Shinohara T., Barrett D. J., Wiggert B., Chader G. J., Nickerson J. M. Retinal photoreceptor neurons and pinealocytes accumulate mRNA for interphotoreceptor retinoid-binding protein (IRBP). FEBS Lett. 1986 Nov 10;208(1):133–137. doi: 10.1016/0014-5793(86)81547-2. [DOI] [PubMed] [Google Scholar]