Abstract
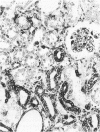
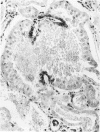
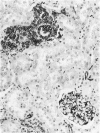
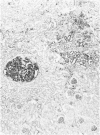
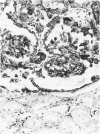
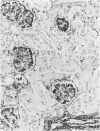
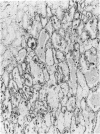
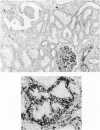
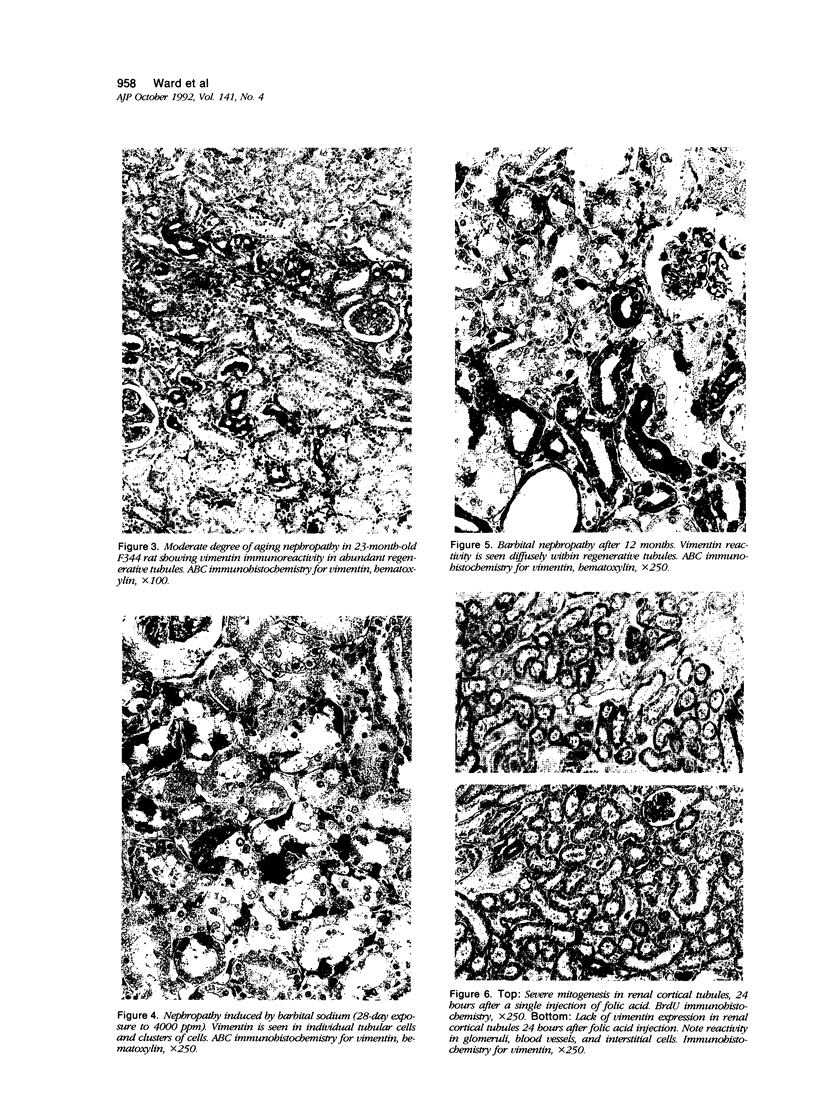
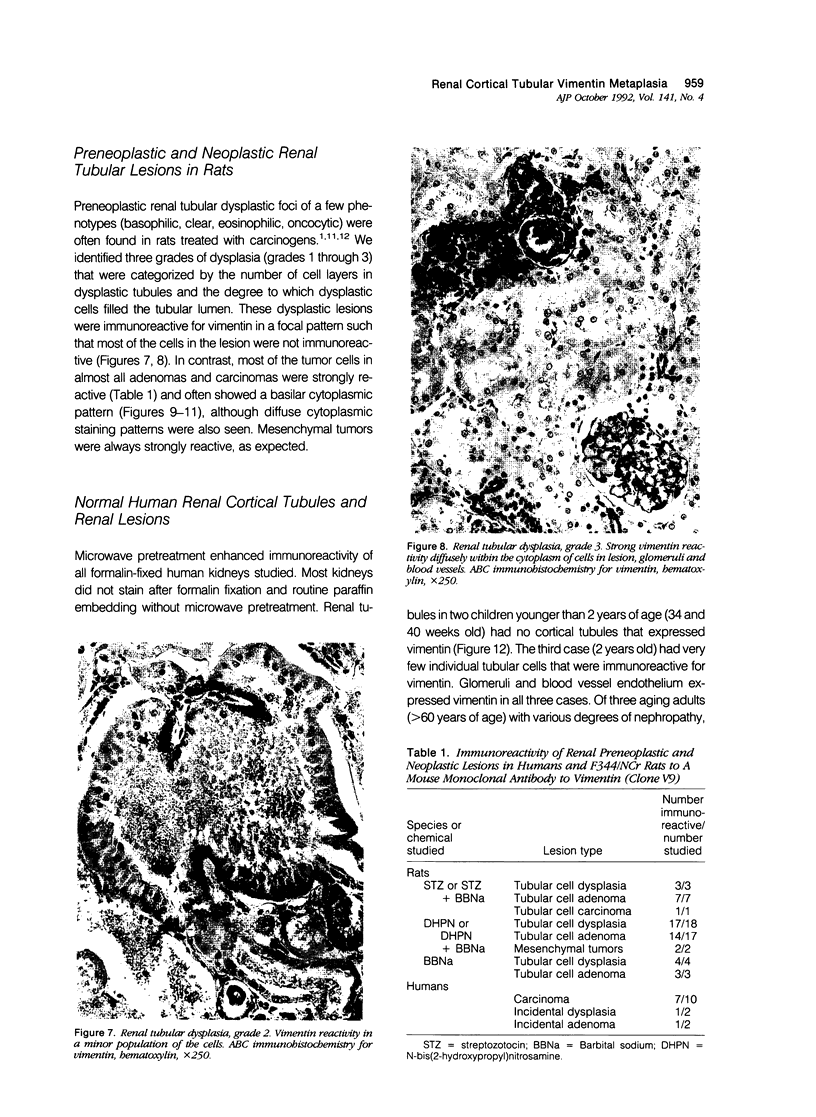
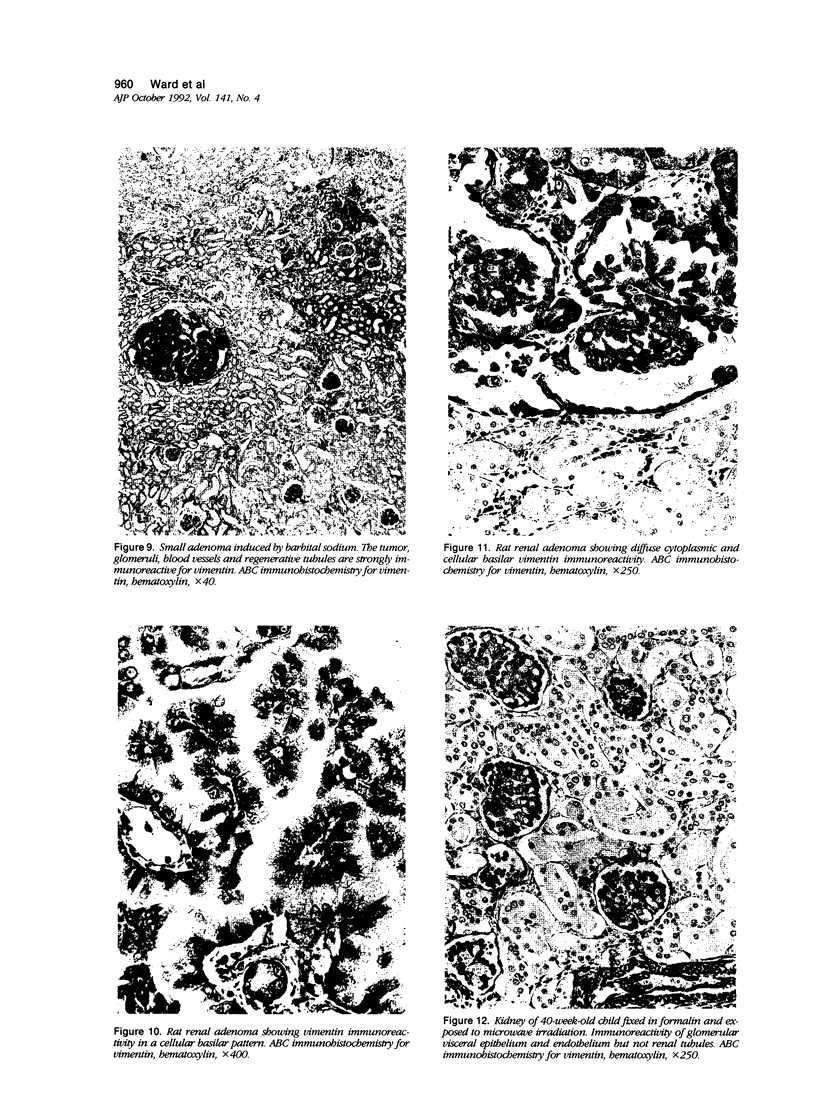
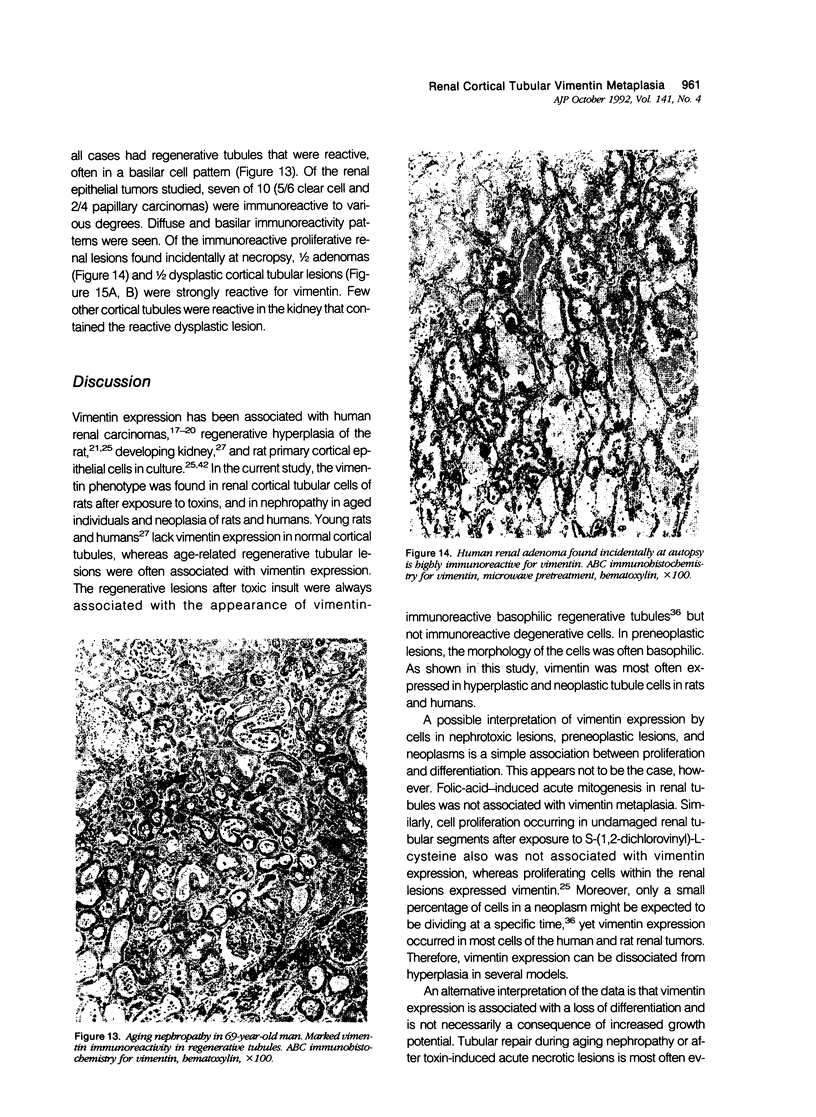
Vimentin expression was studied immunohistochemically in renal cortical tubules of untreated male rats of various ages, rats exposed to toxins (barbital sodium, folic acid) and carcinogens (streptozotocin, N-bis(2-hydroxypropyl)nitrosamine, barbital sodium, and in humans of various ages with or without renal epithelial tumors. Fetal, neonatal, and young adult rats did not express vimentin in renal cortical tubules. Regenerative renal tubular lesions from rats with aging nephropathy and from rats with toxic nephropathy both expressed vimentin. Mitogenic lesions induced by folic acid at 24 hours, however, were not immunoreactive for vimentin. Carcinogen-induced preneoplastic renal cortical tubular lesions in rats were most often focally immunoreactive whereas strong vimentin expression was found in almost all induced renal tumors. In kidneys of three children (younger than 2 years of age), vimentin was not found in renal cortical tubular cells except in rare individual cells in one case. Vimentin was abundant in basophilic regenerative tubules in kidneys of aged individuals, however. Most (7/10) human renal carcinomas and latent preneoplastic or neoplastic renal tubular lesions found incidentally at autopsy (2/4) showed vimentin expression. The authors suggest that the switching to vimentin expression in phenotypically normal renal cortical tubular cells in rats and humans, which do not usually express the intermediate filament protein vimentin, should be considered vimentin metaplasia. Vimentin expression is dissociated from increased cell proliferation in hyperplastic and neoplastic lesions, however. Instead the degree of dedifferentiation of the tubule cells and changes in phenotype were associated with vimentin expression.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azumi N., Battifora H. The distribution of vimentin and keratin in epithelial and nonepithelial neoplasms. A comprehensive immunohistochemical study on formalin- and alcohol-fixed tumors. Am J Clin Pathol. 1987 Sep;88(3):286–296. doi: 10.1093/ajcp/88.3.286. [DOI] [PubMed] [Google Scholar]
- Bachmann S., Kriz W., Kuhn C., Franke W. W. Differentiation of cell types in the mammalian kidney by immunofluorescence microscopy using antibodies to intermediate filament proteins and desmoplakins. Histochemistry. 1983;77(3):365–394. doi: 10.1007/BF00490899. [DOI] [PubMed] [Google Scholar]
- Baserga R., Thatcher D., Marzi D. Cell proliferation in mouse kidney after a single injection of folic acid. Lab Invest. 1968 Jul;19(1):92–96. [PubMed] [Google Scholar]
- Ben-Ze'ev A. The role of changes in cell shape and contacts in the regulation of cytoskeleton expression during differentiation. J Cell Sci Suppl. 1987;8:293–312. doi: 10.1242/jcs.1987.supplement_8.16. [DOI] [PubMed] [Google Scholar]
- Dezso B., Rady P., Morocz I., Varga E., Gomba S., Poulsen K., Kertai P. Morphological and immunohistochemical characteristics of dimethylnitrosamine-induced malignant mesenchymal renal tumor in F-344 rats. J Cancer Res Clin Oncol. 1990;116(4):372–378. doi: 10.1007/BF01612920. [DOI] [PubMed] [Google Scholar]
- Dierick A. M., Praet M., Roels H., Verbeeck P., Robyns C., Oosterlinck W. Vimentin expression of renal cell carcinoma in relation to DNA content and histological grading: a combined light microscopic, immunocytochemical and cytophotometrical analysis. Histopathology. 1991 Apr;18(4):315–322. doi: 10.1111/j.1365-2559.1991.tb00852.x. [DOI] [PubMed] [Google Scholar]
- Dietrich D. R., Swenberg J. A. Preneoplastic lesions in rodent kidney induced spontaneously or by non-genotoxic agents: predictive nature and comparison to lesions induced by genotoxic carcinogens. Mutat Res. 1991 Jun;248(2):239–260. doi: 10.1016/0027-5107(91)90060-2. [DOI] [PubMed] [Google Scholar]
- Drinkwater N. R., Bennett L. M. Genetic control of carcinogenesis in experimental animals. Prog Exp Tumor Res. 1991;33:1–20. doi: 10.1159/000419242. [DOI] [PubMed] [Google Scholar]
- Ekblom P. Developmentally regulated conversion of mesenchyme to epithelium. FASEB J. 1989 Aug;3(10):2141–2150. doi: 10.1096/fasebj.3.10.2666230. [DOI] [PubMed] [Google Scholar]
- Gold L. S., Slone T. H., Manley N. B., Bernstein L. Target organs in chronic bioassays of 533 chemical carcinogens. Environ Health Perspect. 1991 Jun;93:233–246. doi: 10.1289/ehp.9193233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossrau R., Günther T., Graf R. Enhancement of gentamicin-induced nephrotoxicity by Mg deficiency in non-pregnant rats. Morphological, enzyme histochemical and immunohistochemical studies. Histochemistry. 1989;90(6):489–496. doi: 10.1007/BF00494361. [DOI] [PubMed] [Google Scholar]
- Gossrau R., Günther T., Merker H. J., Graf R. Enhancement of maternal and fetal nephrotoxicity of salicylate by zinc deficiency. Morphological, enzyme histochemical and immunohistochemical studies. Histochemistry. 1988;89(1):81–90. doi: 10.1007/BF00496589. [DOI] [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M. Monoclonal antibodies to human intermediate filament proteins. II. Distribution of filament proteins in normal human tissues. Am J Pathol. 1984 Feb;114(2):309–321. [PMC free article] [PubMed] [Google Scholar]
- Gröne H. J., Weber K., Gröne E., Helmchen U., Osborn M. Coexpression of keratin and vimentin in damaged and regenerating tubular epithelia of the kidney. Am J Pathol. 1987 Oct;129(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- Hagiwara A., Diwan B. A., Ward J. M. Barbital sodium, a tumor promoter for kidney tubules, urinary bladder, and liver of the F344 rat, induces persistent increases in levels of DNA synthesis in renal tubules but not in urinary bladder epithelium or hepatocytes. Fundam Appl Toxicol. 1989 Aug;13(2):332–340. doi: 10.1016/0272-0590(89)90269-8. [DOI] [PubMed] [Google Scholar]
- Hard G. C. Pathology of tumours in laboratory animals. Tumours of the rat. Tumours of the kidney, renal pelvis and ureter. IARC Sci Publ. 1990;(99):301–344. [PubMed] [Google Scholar]
- Hatzinger P. B., Chen Q., Dong L. Q., Stevens J. L. Alterations in intermediate filament proteins in rat kidney proximal tubule epithelial cells. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1316–1322. doi: 10.1016/s0006-291x(88)81018-0. [DOI] [PubMed] [Google Scholar]
- Hiasa Y., Ito N. Experimental induction of renal tumors. Crit Rev Toxicol. 1987;17(4):279–343. doi: 10.3109/10408448709029325. [DOI] [PubMed] [Google Scholar]
- Holthöfer H., Miettinen A., Lehto V. P., Lehtonen E., Virtanen I. Expression of vimentin and cytokeratin types of intermediate filament proteins in developing and adult human kidneys. Lab Invest. 1984 May;50(5):552–559. [PubMed] [Google Scholar]
- Holthöfer H., Miettinen A., Paasivuo R., Lehto V. P., Linder E., Alfthan O., Virtanen I. Cellular origin and differentiation of renal carcinomas. A fluorescence microscopic study with kidney-specific antibodies, antiintermediate filament antibodies, and lectins. Lab Invest. 1983 Sep;49(3):317–326. [PubMed] [Google Scholar]
- Konishi N., Diwan B. A., Ward J. M. Amelioration of sodium barbital-induced nephropathy and regenerative tubular hyperplasia after a single injection of streptozotocin does not abolish the renal tumor promoting effect of barbital sodium in male F344/NC4 rats. Carcinogenesis. 1990 Dec;11(12):2149–2156. doi: 10.1093/carcin/11.12.2149. [DOI] [PubMed] [Google Scholar]
- Konishi N., Kitahori Y., Shimoyama T., Lin J. C., Hiasa Y. Monoclonal antibody against rat renal cell tumor-associated antigen as a new tool for the analysis of renal tumorigenesis. Jpn J Cancer Res. 1989 Aug;80(8):771–777. doi: 10.1111/j.1349-7006.1989.tb01713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi N., Ward J. M. Increased levels of DNA synthesis in hyperplastic renal tubules of aging nephropathy in female F344/NCr rats. Vet Pathol. 1989 Jan;26(1):6–10. doi: 10.1177/030098588902600102. [DOI] [PubMed] [Google Scholar]
- Laurent G., Toubeau G., Heuson-Stiennon J. A., Tulkens P., Maldague P. Kidney tissue repair after nephrotoxic injury: biochemical and morphological characterization. Crit Rev Toxicol. 1988;19(2):147–183. doi: 10.3109/10408448809014903. [DOI] [PubMed] [Google Scholar]
- Lehtonen E., Virtanen I., Saxén L. Reorganization of intermediate filament cytoskeleton in induced metanephric mesenchyme cells is independent of tubule morphogenesis. Dev Biol. 1985 Apr;108(2):481–490. doi: 10.1016/0012-1606(85)90051-x. [DOI] [PubMed] [Google Scholar]
- Moll R., Hage C., Thoenes W. Expression of intermediate filament proteins in fetal and adult human kidney: modulations of intermediate filament patterns during development and in damaged tissue. Lab Invest. 1991 Jul;65(1):74–86. [PubMed] [Google Scholar]
- Oberley T. D., Gonzalez A., Lauchner L. J., Oberley L. W., Li J. J. Characterization of early kidney lesions in estrogen-induced tumors in the Syrian hamster. Cancer Res. 1991 Apr 1;51(7):1922–1929. [PubMed] [Google Scholar]
- Ohmori T., Murata Y., Kitamura H. An etiological study on renal adenomas: with some references to dysplastic tubular lesions and adenocarcinomas in autopsy and surgery cases. Acta Pathol Jpn. 1982 Jul;32(4):585–593. [PubMed] [Google Scholar]
- Osborn M., Debus E., Weber K. Monoclonal antibodies specific for vimentin. Eur J Cell Biol. 1984 May;34(1):137–143. [PubMed] [Google Scholar]
- Pierce G. B., Speers W. C. Tumors as caricatures of the process of tissue renewal: prospects for therapy by directing differentiation. Cancer Res. 1988 Apr 15;48(8):1996–2004. [PubMed] [Google Scholar]
- Pitz S., Moll R., Störkel S., Thoenes W. Expression of intermediate filament proteins in subtypes of renal cell carcinomas and in renal oncocytomas. Distinction of two classes of renal cell tumors. Lab Invest. 1987 Jun;56(6):642–653. [PubMed] [Google Scholar]
- Potter V. R. Phenotypic diversity in experimental hepatomas: the concept of partially blocked ontogeny. The 10th Walter Hubert Lecture. Br J Cancer. 1978 Jul;38(1):1–23. doi: 10.1038/bjc.1978.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S. R., Key M. E., Kalra K. L. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem. 1991 Jun;39(6):741–748. doi: 10.1177/39.6.1709656. [DOI] [PubMed] [Google Scholar]
- Shimokama T., Watanabe T. Monoclonal antibodies to rat renal tissue: an approach to the immunohistological analysis of the nephron-collecting duct system, and ultra-structural localization of antigens. Histochem J. 1991 Jan;23(1):13–21. doi: 10.1007/BF01886503. [DOI] [PubMed] [Google Scholar]
- Stevens J. L., Jones T. W. The role of damage and proliferation in renal carcinogenesis. Toxicol Lett. 1990 Sep;53(1-2):121–126. doi: 10.1016/0378-4274(90)90105-u. [DOI] [PubMed] [Google Scholar]
- Suzuki M., Nikaido T., Ikegami M., Kikuchi Y., Takasaki S., Furusato M., Aizawa S. Renal adenoma. Clinicopathological and histochemical studies. Acta Pathol Jpn. 1989 Nov;39(11):731–736. [PubMed] [Google Scholar]
- Tsuda H., Hacker H. J., Katayama H., Masui T., Ito N., Bannasch P. Correlative histochemical studies on preneoplastic and neoplastic lesions in the kidney of rats treated with nitrosamines. Virchows Arch B Cell Pathol Incl Mol Pathol. 1986;51(5):385–404. doi: 10.1007/BF02899047. [DOI] [PubMed] [Google Scholar]
- Wallin A., Zhang G., Jones T. W., Jaken S., Stevens J. L. Mechanism of the nephrogenic repair response. Studies on proliferation and vimentin expression after 35S-1,2-dichlorovinyl-L-cysteine nephrotoxicity in vivo and in cultured proximal tubule epithelial cells. Lab Invest. 1992 Apr;66(4):474–484. [PubMed] [Google Scholar]
- Ward J. M., Rehm S. Applications of immunohistochemistry in rodent tumor pathology. Exp Pathol. 1990;40(4):301–312. doi: 10.1016/s0232-1513(11)80317-8. [DOI] [PubMed] [Google Scholar]
- Xipell J. M. The incidence of benign renal nodules (a clinicopathologic study). J Urol. 1971 Oct;106(4):503–506. doi: 10.1016/s0022-5347(17)61327-2. [DOI] [PubMed] [Google Scholar]