Abstract
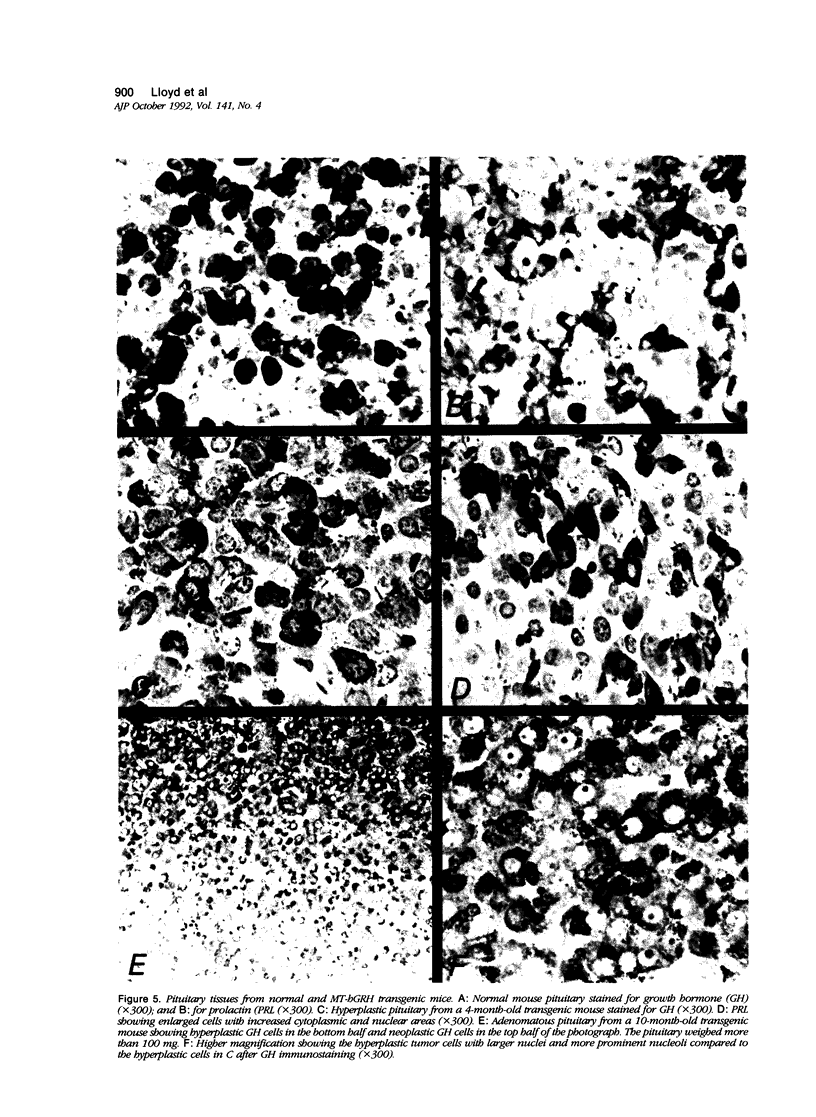
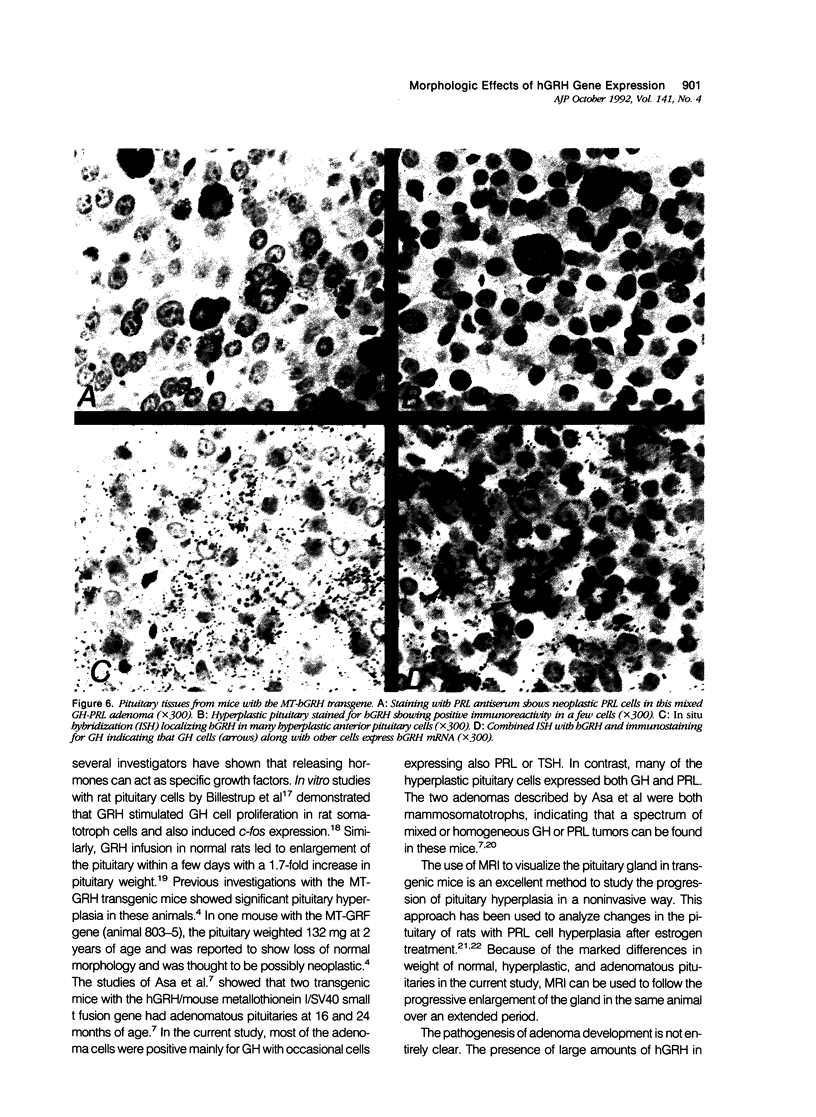
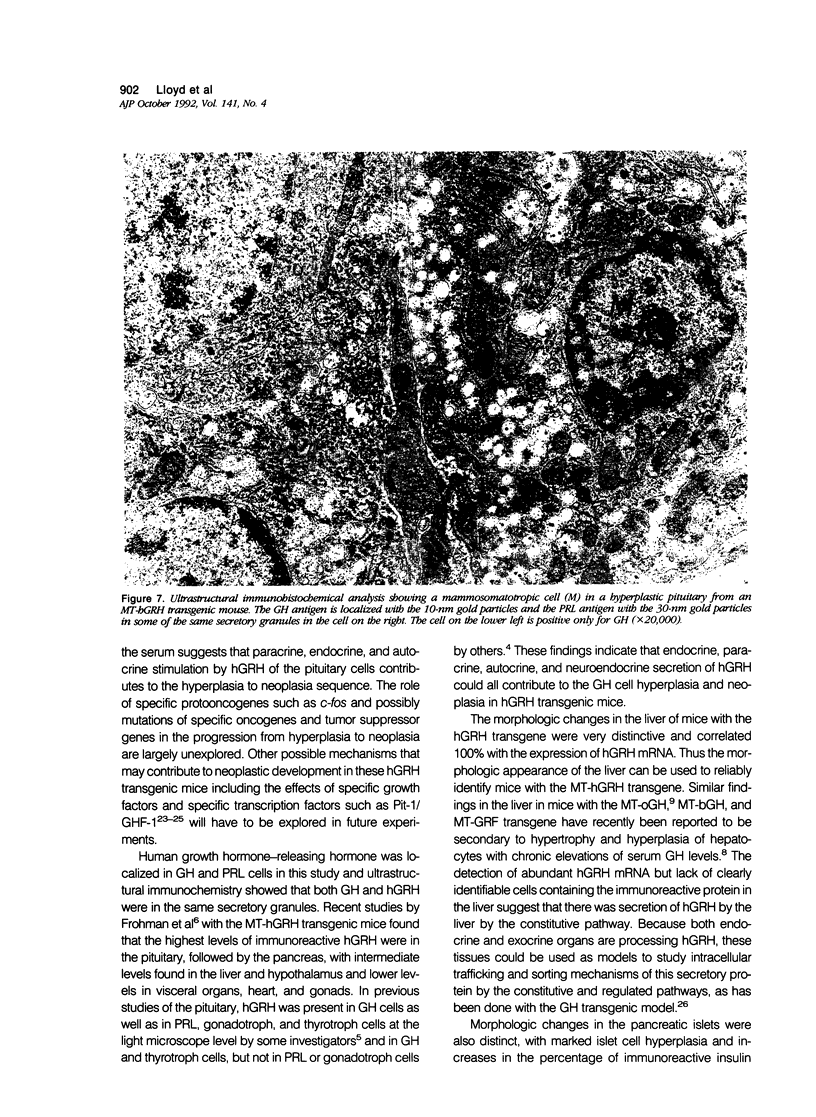
Morphologic changes in the pituitary, liver, and pancreas of mice with the metallothionein-human growth hormone--releasing hormone (MT-hGRH) transgene were analyzed by in situ hybridization histochemistry (ISH). There was progression from somatotroph hyperplasia to neoplasia in pituitaries of transgenic mice. Pituitary neoplasms were present between 9 to 12 months of age in some mice. Magnetic resonance imaging (MRI) readily identified enlarged pituitaries in MT-hGRH transgenic mice. Serum mouse GH and hGRH levels were marked elevated in MT-hGRH transgenic mice. In situ hybridization histochemistry showed mRNA for hGRH in liver, pituitary, pancreas, spleen, and in most other tissues examined. Combined ISH and immunohistochemistry in the pituitary gland showed that some of the GH cells also produced hGRH, and ultrastructural immunohistochemical analysis of pituitaries showed that GH and hGRH were localized in the same cell and within the same secretory granules. Liver cells of MT-hGRH transgenic mice showed evidence of hypertrophy, and the pancreatic islets were hyperplastic with significant increases in the islet cell areas. The morphologic changes in the liver were distinctive enough to separate control littermates from MT-hGRH transgenic mice in all cases. The enlarged pancreatic islets had increased numbers of insulin-producing cells. Immunoreactive hGRH and hGRH mRNA were both localized in islet cells, and an intense hybridization signal of hGRH mRNA, but only weak staining for hGRH protein, were detected in the liver of transgenic mice. These results indicate that excessive hGRH production leads to distinct morphologic changes in various organs in MT-hGRH transgenic mice and that there is temporal progression from hyperplasia to adenomatous somatotrophs in pituitaries with chronic stimulation by hGRH that involves paracrine, endocrine, and autocrine mechanisms.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asa S. L., Kovacs K., Stefaneanu L., Horvath E., Billestrup N., Gonzalez-Manchon C., Vale W. Pituitary mammosomatotroph adenomas develop in old mice transgenic for growth hormone-releasing hormone. Proc Soc Exp Biol Med. 1990 Mar;193(3):232–235. doi: 10.3181/00379727-193-3-rc1. [DOI] [PubMed] [Google Scholar]
- Asa S. L., Scheithauer B. W., Bilbao J. M., Horvath E., Ryan N., Kovacs K., Randall R. V., Laws E. R., Jr, Singer W., Linfoot J. A. A case for hypothalamic acromegaly: a clinicopathological study of six patients with hypothalamic gangliocytomas producing growth hormone-releasing factor. J Clin Endocrinol Metab. 1984 May;58(5):796–803. doi: 10.1210/jcem-58-5-796. [DOI] [PubMed] [Google Scholar]
- Billestrup N., Mitchell R. L., Vale W., Verma I. M. Growth hormone-releasing factor induces c-fos expression in cultured primary pituitary cells. Mol Endocrinol. 1987 Apr;1(4):300–305. doi: 10.1210/mend-1-4-300. [DOI] [PubMed] [Google Scholar]
- Billestrup N., Swanson L. W., Vale W. Growth hormone-releasing factor stimulates proliferation of somatotrophs in vitro. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6854–6857. doi: 10.1073/pnas.83.18.6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brar A. K., Brinster R. L., Frohman L. A. Immunohistochemical analysis of human growth hormone-releasing hormone gene expression in transgenic mice. Endocrinology. 1989 Aug;125(2):801–809. doi: 10.1210/endo-125-2-801. [DOI] [PubMed] [Google Scholar]
- Camper S. A., Saunders T. L., Katz R. W., Reeves R. H. The Pit-1 transcription factor gene is a candidate for the murine Snell dwarf mutation. Genomics. 1990 Nov;8(3):586–590. doi: 10.1016/0888-7543(90)90050-5. [DOI] [PubMed] [Google Scholar]
- Castrillo J. L., Theill L. E., Karin M. Function of the homeodomain protein GHF1 in pituitary cell proliferation. Science. 1991 Jul 12;253(5016):197–199. doi: 10.1126/science.1677216. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Frohman L. A., Downs T. R., Kashio Y., Brinster R. L. Tissue distribution and molecular heterogeneity of human growth hormone-releasing factor in the transgenic mouse. Endocrinology. 1990 Nov;127(5):2149–2156. doi: 10.1210/endo-127-5-2149. [DOI] [PubMed] [Google Scholar]
- Frohman L. A., Downs T. R. Measurement of growth hormone-releasing factor. Methods Enzymol. 1986;124:371–389. doi: 10.1016/0076-6879(86)24029-x. [DOI] [PubMed] [Google Scholar]
- Furth J. Pituitary cybernetics and neoplasia. Harvey Lect. 1969;63:47–71. [PubMed] [Google Scholar]
- Hammer R. E., Brinster R. L., Rosenfeld M. G., Evans R. M., Mayo K. E. Expression of human growth hormone-releasing factor in transgenic mice results in increased somatic growth. 1985 May 30-Jun 5Nature. 315(6018):413–416. doi: 10.1038/315413a0. [DOI] [PubMed] [Google Scholar]
- Joubert D., Benlot C., Lagoguey A., Garnier P., Brandi A. M., Gautron J. P., Legrand J. C., Peillon F. Normal and growth hormone (GH)-secreting adenomatous human pituitaries release somatostatin and GH-releasing hormone. J Clin Endocrinol Metab. 1989 Mar;68(3):572–577. doi: 10.1210/jcem-68-3-572. [DOI] [PubMed] [Google Scholar]
- Lloyd R. V., Anagnostou D., Cano M., Barkan A. L., Chandler W. F. Analysis of mammosomatotropic cells in normal and neoplastic human pituitary tissues by the reverse hemolytic plaque assay and immunocytochemistry. J Clin Endocrinol Metab. 1988 Jun;66(6):1103–1110. doi: 10.1210/jcem-66-6-1103. [DOI] [PubMed] [Google Scholar]
- Lloyd R. V., Cano M., Chandler W. F., Barkan A. L., Horvath E., Kovacs K. Human growth hormone and prolactin secreting pituitary adenomas analyzed by in situ hybridization. Am J Pathol. 1989 Mar;134(3):605–613. [PMC free article] [PubMed] [Google Scholar]
- Lloyd R. V., Cano M., Landefeld T. D. The effects of estrogens on tumor growth and on prolactin and growth hormone mRNA expression in rat pituitary tissues. Am J Pathol. 1988 Nov;133(2):397–406. [PMC free article] [PubMed] [Google Scholar]
- Lloyd R. V., Iacangelo A., Eiden L. E., Cano M., Jin L., Grimes M. Chromogranin A and B messenger ribonucleic acids in pituitary and other normal and neoplastic human endocrine tissues. Lab Invest. 1989 Apr;60(4):548–556. [PubMed] [Google Scholar]
- Lloyd R. V., Jin L., Fields K., Kulig E. Regulation of prolactin gene expression in a DMBA-estrogen-induced transplantable rat pituitary tumor. Am J Pathol. 1990 Dec;137(6):1525–1537. [PMC free article] [PubMed] [Google Scholar]
- Lloyd R. V. Pathology of tumours in laboratory animals. Tumours of the rat. Tumours of the pituitary gland. IARC Sci Publ. 1990;(99):499–537. [PubMed] [Google Scholar]
- Mayo K. E., Cerelli G. M., Lebo R. V., Bruce B. D., Rosenfeld M. G., Evans R. M. Gene encoding human growth hormone-releasing factor precursor: structure, sequence, and chromosomal assignment. Proc Natl Acad Sci U S A. 1985 Jan;82(1):63–67. doi: 10.1073/pnas.82.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo K. E., Hammer R. E., Swanson L. W., Brinster R. L., Rosenfeld M. G., Evans R. M. Dramatic pituitary hyperplasia in transgenic mice expressing a human growth hormone-releasing factor gene. Mol Endocrinol. 1988 Jul;2(7):606–612. doi: 10.1210/mend-2-7-606. [DOI] [PubMed] [Google Scholar]
- Melmed S., Braunstein G. D., Horvath E., Ezrin C., Kovacs K. Pathophysiology of acromegaly. Endocr Rev. 1983 Summer;4(3):271–290. doi: 10.1210/edrv-4-3-271. [DOI] [PubMed] [Google Scholar]
- Orian J. M., Lee C. S., Weiss L. M., Brandon M. R. The expression of a metallothionein-ovine growth hormone fusion gene in transgenic mice does not impair fertility but results in pathological lesions in the liver. Endocrinology. 1989 Jan;124(1):455–463. doi: 10.1210/endo-124-1-455. [DOI] [PubMed] [Google Scholar]
- Quaife C. J., Mathews L. S., Pinkert C. A., Hammer R. E., Brinster R. L., Palmiter R. D. Histopathology associated with elevated levels of growth hormone and insulin-like growth factor I in transgenic mice. Endocrinology. 1989 Jan;124(1):40–48. doi: 10.1210/endo-124-1-40. [DOI] [PubMed] [Google Scholar]
- Sano T., Asa S. L., Kovacs K. Growth hormone-releasing hormone-producing tumors: clinical, biochemical, and morphological manifestations. Endocr Rev. 1988 Aug;9(3):357–373. doi: 10.1210/edrv-9-3-357. [DOI] [PubMed] [Google Scholar]
- Simmons D. M., Voss J. W., Ingraham H. A., Holloway J. M., Broide R. S., Rosenfeld M. G., Swanson L. W. Pituitary cell phenotypes involve cell-specific Pit-1 mRNA translation and synergistic interactions with other classes of transcription factors. Genes Dev. 1990 May;4(5):695–711. doi: 10.1101/gad.4.5.695. [DOI] [PubMed] [Google Scholar]
- Stefaneanu L., Kovacs K., Horvath E., Asa S. L., Losinski N. E., Billestrup N., Price J., Vale W. Adenohypophysial changes in mice transgenic for human growth hormone-releasing factor: a histological, immunocytochemical, and electron microscopic investigation. Endocrinology. 1989 Nov;125(5):2710–2718. doi: 10.1210/endo-125-5-2710. [DOI] [PubMed] [Google Scholar]
- Thorner M. O., Perryman R. L., Cronin M. J., Rogol A. D., Draznin M., Johanson A., Vale W., Horvath E., Kovacs K. Somatotroph hyperplasia. Successful treatment of acromegaly by removal of a pancreatic islet tumor secreting a growth hormone-releasing factor. J Clin Invest. 1982 Nov;70(5):965–977. doi: 10.1172/JCI110708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trahair J. F., Neutra M. R., Gordon J. I. Use of transgenic mice to study the routing of secretory proteins in intestinal epithelial cells: analysis of human growth hormone compartmentalization as a function of cell type and differentiation. J Cell Biol. 1989 Dec;109(6 Pt 2):3231–3242. doi: 10.1083/jcb.109.6.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nesselrooij J. H., Bruijntjes J. P., van Garderen-Hoetmer A., Tillapaugh-Fay G. M., Feron V. J. Magnetic resonance imaging compared with hormonal effects and histopathology of estrogen-induced pituitary lesions in the rat. Carcinogenesis. 1991 Feb;12(2):289–297. doi: 10.1093/carcin/12.2.289. [DOI] [PubMed] [Google Scholar]
- van Nesselrooij J. H., Szeverenyi N. M., Tillapaugh-Fay G. M., Hendriksen F. G. Gadolinium-DTPA-enhanced and digitally subtracted magnetic resonance imaging of estrogen-induced pituitary lesions in rats: correlation with pituitary anatomy. Magn Reson Imaging. 1990;8(5):525–533. doi: 10.1016/0730-725x(90)90129-p. [DOI] [PubMed] [Google Scholar]