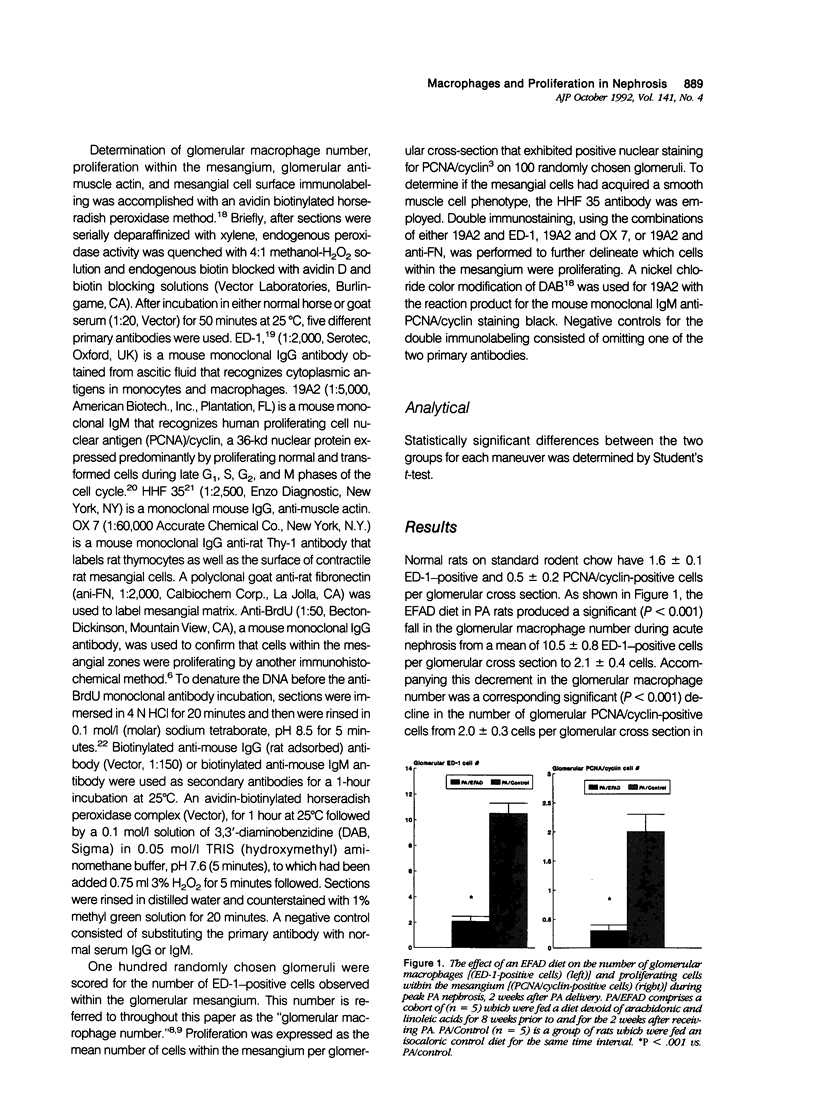
Abstract
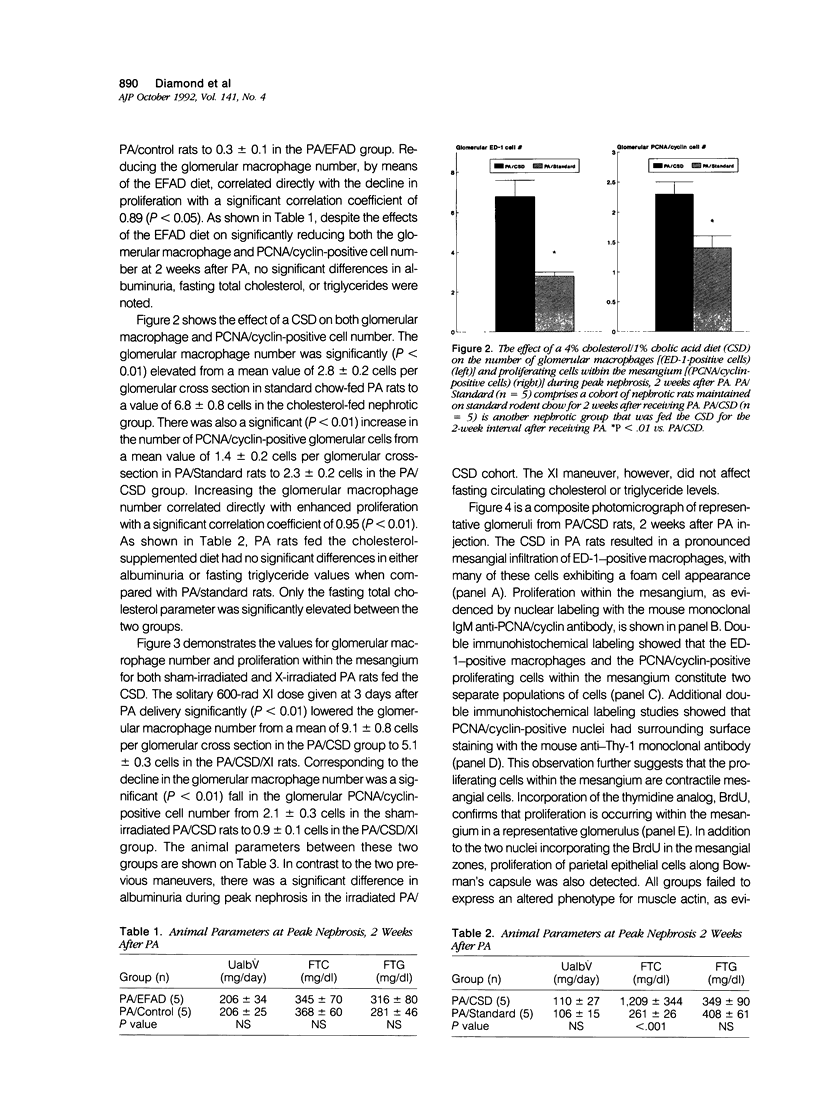
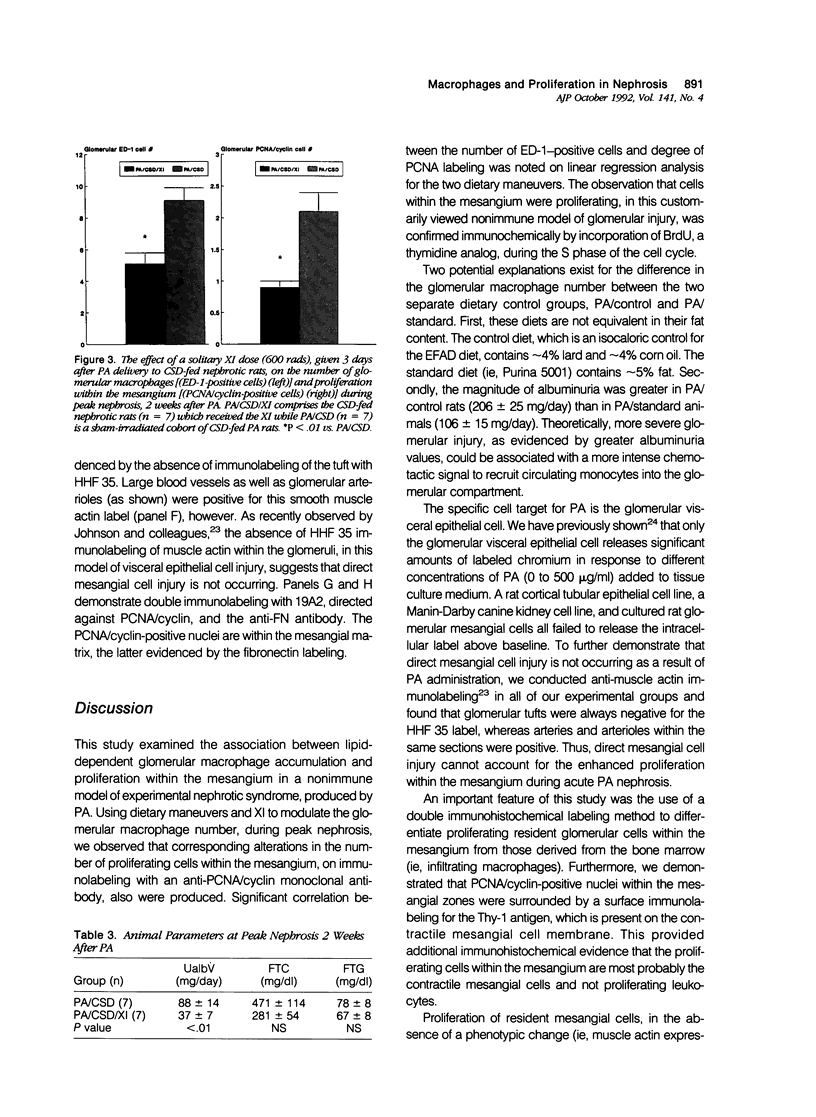
Mesangial cell proliferation, which is a harbinger of glomerulosclerosis, occurs in both immune and nonimmune glomerulopathies. The proximity of infiltrating glomerular macrophages to the contractile mesangial cells during acute puromycin aminonucleoside (PA) nephrosis suggests the possibility of a paracrine effect on mesangial cell growth. To test this, three maneuvers to either raise or lower the glomerular macrophage number during acute PA nephrosis (2 weeks after PA) were employed: 1) an essential fatty acid-deficient (EFAD) diet; 2) a cholesterol-supplemented diet (CSD); and 3) a single dose (600 rad) whole-body X-irradiation (XI) given to CSD-fed PA rats. Both the glomerular macrophage number and proliferation within the mesangium were evaluated immunohistochemically with ED-1, a mouse monoclonal anti-rat macrophage label, and 19A2, a mouse monoclonal anti-proliferating cell nuclear antigen (PCNA)/cyclin antibody, respectively. Immunohistochemical detection of 5'-bromo-2'-deoxyuridine (BrdU) incorporation confirmed that proliferation was occurring within the mesangial zones. The EFAD diet significantly reduced both the glomerular macrophage and PCNA/cyclin-positive cell number at 2 weeks after PA with a positive correlation (r = 0.89, P < 0.05). The CSD maneuver significantly increased both the glomerular macrophage and PCNA/cyclin cell number with a strong degree of correlation (r = 0.95, P < 0.01). X-irradiation administered to CSD-fed PA rats significantly lowered both the glomerular macrophage and PCNA/cyclin-positive cell number at 2 weeks. In all groups, the glomerular tufts did not express muscle actin using HHF 35, a specific immunolabel, suggesting that the proliferation in this model is not related to direct mesangial cell injury. This study shows that maneuvers that modulate the glomerular macrophage number are also associated with corresponding changes in the number of proliferating cells within the mesangium, suggesting a paracrine growth stimulation by the infiltrating macrophage during acute PA nephrosis. The infiltrating glomerular macrophage may be an effector mechanism for the propagation of initial glomerular injury to glomerulosclerosis by augmenting mesangial cell proliferation early in the course of this nonimmune progressive glomerulopathy.
Full text
PDF



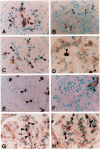
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Diamond J. R., Anderson S. Irreversible tubulointerstitial damage associated with chronic aminonucleoside nephrosis. Amelioration by angiotensin I converting enzyme inhibition. Am J Pathol. 1990 Dec;137(6):1323–1332. [PMC free article] [PubMed] [Google Scholar]
- Diamond J. R., Karnovsky M. J. Focal and segmental glomerulosclerosis following a single intravenous dose of puromycin aminonucleoside. Am J Pathol. 1986 Mar;122(3):481–487. [PMC free article] [PubMed] [Google Scholar]
- Diamond J. R., Karnovsky M. J. Focal and segmental glomerulosclerosis: analogies to atherosclerosis. Kidney Int. 1988 May;33(5):917–924. doi: 10.1038/ki.1988.87. [DOI] [PubMed] [Google Scholar]
- Diamond J. R., Pesek-Diamond I. Sublethal X-irradiation during acute puromycin nephrosis prevents late renal injury: role of macrophages. Am J Physiol. 1991 Jun;260(6 Pt 2):F779–F786. doi: 10.1152/ajprenal.1991.260.6.F779. [DOI] [PubMed] [Google Scholar]
- Diamond J. R., Pesek I. Glomerular tumor necrosis factor and interleukin 1 during acute aminonucleoside nephrosis. An immunohistochemical study. Lab Invest. 1991 Jan;64(1):21–28. [PubMed] [Google Scholar]
- Diamond J. R., Pesek I., McCarter M. D., Karnovsky M. J. Altered functional characteristics of rat macrophages during nephrosis. Synergistic effects of hypercholesterolemia. Am J Pathol. 1989 Oct;135(4):711–718. [PMC free article] [PubMed] [Google Scholar]
- Diamond J. R., Pesek I., Ruggieri S., Karnovsky M. J. Essential fatty acid deficiency during acute puromycin nephrosis ameliorates late renal injury. Am J Physiol. 1989 Nov;257(5 Pt 2):F798–F807. doi: 10.1152/ajprenal.1989.257.5.F798. [DOI] [PubMed] [Google Scholar]
- Dijkstra C. D., Döpp E. A., Joling P., Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology. 1985 Mar;54(3):589–599. [PMC free article] [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M. Monoclonal antibodies to human intermediate filament proteins. II. Distribution of filament proteins in normal human tissues. Am J Pathol. 1984 Feb;114(2):309–321. [PMC free article] [PubMed] [Google Scholar]
- Harris K. P., Lefkowith J. B., Klahr S., Schreiner G. F. Essential fatty acid deficiency ameliorates acute renal dysfunction in the rat after the administration of the aminonucleoside of puromycin. J Clin Invest. 1990 Oct;86(4):1115–1123. doi: 10.1172/JCI114816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffer F., Saunders C., Shultz P., Throckmorton D., Weinshell E., Abboud H. E. Regulation of mesangial cell growth by polypeptide mitogens. Inhibitory role of transforming growth factor beta. Am J Pathol. 1989 Aug;135(2):261–269. [PMC free article] [PubMed] [Google Scholar]
- Johnson R. J., Garcia R. L., Pritzl P., Alpers C. E. Platelets mediate glomerular cell proliferation in immune complex nephritis induced by anti-mesangial cell antibodies in the rat. Am J Pathol. 1990 Feb;136(2):369–374. [PMC free article] [PubMed] [Google Scholar]
- Johnson R. J., Iida H., Alpers C. E., Majesky M. W., Schwartz S. M., Pritzi P., Gordon K., Gown A. M. Expression of smooth muscle cell phenotype by rat mesangial cells in immune complex nephritis. Alpha-smooth muscle actin is a marker of mesangial cell proliferation. J Clin Invest. 1991 Mar;87(3):847–858. doi: 10.1172/JCI115089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klahr S., Schreiner G., Ichikawa I. The progression of renal disease. N Engl J Med. 1988 Jun 23;318(25):1657–1666. doi: 10.1056/NEJM198806233182505. [DOI] [PubMed] [Google Scholar]
- Lacy E. R., Kuwayama H., Cowart K. S., King J. S., Deutz A. H., Sistrunk S. A rapid, accurate, immunohistochemical method to label proliferating cells in the digestive tract. A comparison with tritiated thymidine. Gastroenterology. 1991 Jan;100(1):259–262. doi: 10.1016/0016-5085(91)90610-w. [DOI] [PubMed] [Google Scholar]
- Lefkowith J. B., Schreiner G. Essential fatty acid deficiency depletes rat glomeruli of resident macrophages and inhibits angiotensin II-induced eicosanoid synthesis. J Clin Invest. 1987 Oct;80(4):947–956. doi: 10.1172/JCI113187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett D. H., Ryan J. L., Sterzel R. B. Stimulation of rat mesangial cell proliferation by macrophage interleukin 1. J Immunol. 1983 Dec;131(6):2830–2836. [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Atkins R. C. Glomerular cells and macrophages in the progression of experimental focal and segmental glomerulosclerosis. Am J Pathol. 1989 Apr;134(4):933–945. [PMC free article] [PubMed] [Google Scholar]
- Ogata K., Kurki P., Celis J. E., Nakamura R. M., Tan E. M. Monoclonal antibodies to a nuclear protein (PCNA/cyclin) associated with DNA replication. Exp Cell Res. 1987 Feb;168(2):475–486. doi: 10.1016/0014-4827(87)90020-6. [DOI] [PubMed] [Google Scholar]
- Saito T., Atkins R. C. Contribution of mononuclear leucocytes to the progression of experimental focal glomerular sclerosis. Kidney Int. 1990 Apr;37(4):1076–1083. doi: 10.1038/ki.1990.88. [DOI] [PubMed] [Google Scholar]
- Schreiner G. F., Cotran R. S., Pardo V., Unanue E. R. A mononuclear cell component in experimental immunological glomerulonephritis. J Exp Med. 1978 Feb 1;147(2):369–384. doi: 10.1084/jem.147.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. M., Lewis E. J. Focal segmental glomerular sclerosis: the cellular lesion. Kidney Int. 1985 Dec;28(6):968–974. doi: 10.1038/ki.1985.225. [DOI] [PubMed] [Google Scholar]
- Sterzel R. B., Pabst R. The temporal relationship between glomerular cell proliferation and monocyte infiltration in experimental glomerulonephritis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1982;38(3):337–350. doi: 10.1007/BF02892829. [DOI] [PubMed] [Google Scholar]
- Tsukada T., McNutt M. A., Ross R., Gown A. M. HHF35, a muscle actin-specific monoclonal antibody. II. Reactivity in normal, reactive, and neoplastic human tissues. Am J Pathol. 1987 May;127(2):389–402. [PMC free article] [PubMed] [Google Scholar]
- van Goor H., Fidler V., Weening J. J., Grond J. Determinants of focal and segmental glomerulosclerosis in the rat after renal ablation. Evidence for involvement of macrophages and lipids. Lab Invest. 1991 Jun;64(6):754–765. [PubMed] [Google Scholar]