Abstract
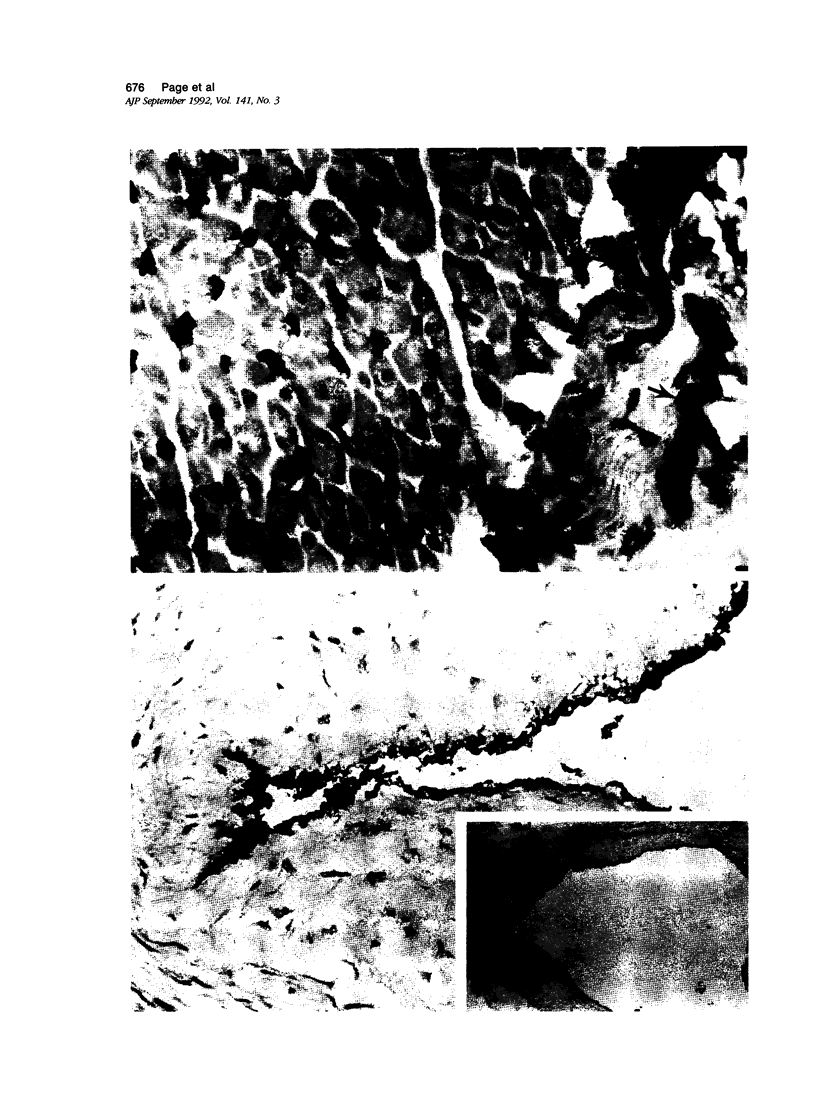
The antigenic status of vascular endothelium from different sites of the normal adult and fetal human cardiovascular system was investigated. Tissues included aorta (n = 9), pulmonary artery (n = 8), coronary artery (n = 6), ventricle/atrium (n = greater than 10), lymph node (n = 2), fetal whole heart (n = 3), and umbilical cord (n = 7). Frozen sections were studied using monoclonal antibodies recognizing endothelial markers (EN4, vWf, Pal-E, and 44G4), vascular adhesion molecules (ICAM-1, ELAM, VCAM, and PECAM), the monocyte/endothelial marker (OKM5), and major histocompatibility complex (MHC) molecules (class I and class II). Results demonstrate that capillary endothelium is phenotypically different from endothelial cells (EC) lining large vessels. Capillary EC strongly express MHC classes I and II, ICAM, and OKM5, which are variably weak to undetectable on large vessels. In contrast, the large vessels strongly express vWf and appear to constitutively express ELAM-1. This suggests that the capillary EC may be more efficient at antigen presentation or more susceptible to immune attack in vivo. Interestingly, normal coronary arteries, unlike all other large vessels, express MHC class II and VCAM molecules. Future studies should concentrate on comparative functional studies between capillary, coronary, and large vessel EC.
Full text
PDF


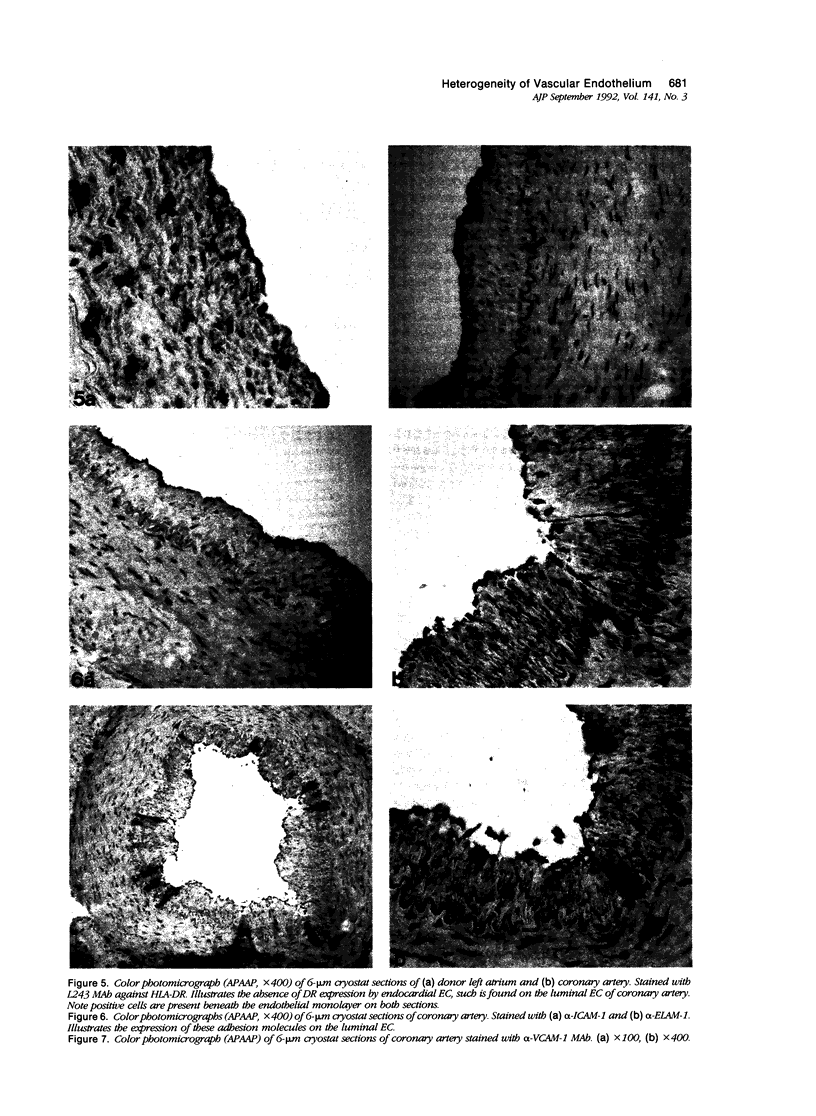


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Mendrick D. L., Cotran R. S., Gimbrone M. A., Jr Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe D. M., Schoen F. J., Rice G. E., Bevilacqua M. P., Ganz P., Pober J. S. Induced expression of endothelial-leukocyte adhesion molecules in human cardiac allografts. Transplantation. 1991 Feb;51(2):537–539. [PubMed] [Google Scholar]
- Cerilli J., Brasile L., Galouzis T., Lempert N., Clarke J. The vascular endothelial cell antigen system. Transplantation. 1985 Mar;39(3):286–289. doi: 10.1097/00007890-198503000-00016. [DOI] [PubMed] [Google Scholar]
- Cui Y. C., Tai P. C., Gatter K. C., Mason D. Y., Spry C. J. A vascular endothelial cell antigen with restricted distribution in human foetal, adult and malignant tissues. Immunology. 1983 May;49(1):183–189. [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Elices M. J., Osborn L., Takada Y., Crouse C., Luhowskyj S., Hemler M. E., Lobb R. R. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990 Feb 23;60(4):577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- Erber W. N., Pinching A. J., Mason D. Y. Immunocytochemical detection of T and B cell populations in routine blood smears. Lancet. 1984 May 12;1(8385):1042–1046. doi: 10.1016/s0140-6736(84)91451-x. [DOI] [PubMed] [Google Scholar]
- Fleming S., Jones D. B. Antigenic heterogeneity of renal endothelium. J Pathol. 1989 Aug;158(4):319–323. doi: 10.1002/path.1711580409. [DOI] [PubMed] [Google Scholar]
- Fukuda Y., Nagura H., Imoto M., Koyama Y. Immunohistochemical studies on structural changes of the hepatic lobules in chronic liver diseases. Am J Gastroenterol. 1986 Dec;81(12):1149–1155. [PubMed] [Google Scholar]
- Gougos A., Letarte M. Identification of a human endothelial cell antigen with monoclonal antibody 44G4 produced against a pre-B leukemic cell line. J Immunol. 1988 Sep 15;141(6):1925–1933. [PubMed] [Google Scholar]
- Hengstenberg C., Rose M. L., Page C., Taylor P. M., Yacoub M. H. Immunocytochemical changes suggestive of damage to endothelial cells during rejection of human cardiac allografts. Transplantation. 1990 May;49(5):895–899. doi: 10.1097/00007890-199005000-00012. [DOI] [PubMed] [Google Scholar]
- Hirschberg H., Bergh O. J., Thorsby E. Antigen-presenting properties of human vascular endothelial cells. J Exp Med. 1980 Aug 1;152(2 Pt 2):249s–255s. [PubMed] [Google Scholar]
- Hirschberg H., Evensen S. A., Henriksen T., Thorsby E. Stimulation of human lymphocytes by allogeneic endothelial cells in vitro. Tissue Antigens. 1974;4(3):257–261. doi: 10.1111/j.1399-0039.1974.tb00249.x. [DOI] [PubMed] [Google Scholar]
- Hirschberg H., Evensen S. A., Henriksen T., Thorsby E. The human mixed lymphocyte-endothelium culture interaction. Transplantation. 1975 Jun;19(6):495–504. doi: 10.1097/00007890-197506000-00008. [DOI] [PubMed] [Google Scholar]
- Hirschberg H., Moen T., Throsby E. Specific destruction of human endothelial cell monolayers by anti-DRw antisera. Transplantation. 1979 Aug;28(2):116–120. doi: 10.1097/00007890-197908000-00009. [DOI] [PubMed] [Google Scholar]
- Hoyer L. W., De los Santos R. P., Hoyer J. R. Antihemophilic factor antigen. Localization in endothelial cells by immunofluorescent microscopy. J Clin Invest. 1973 Nov;52(11):2737–2744. doi: 10.1172/JCI107469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinjo T., Takashi M., Miyake K., Nagura H. Phenotypic heterogeneity of vascular endothelial cells in the human kidney. Cell Tissue Res. 1989 Apr;256(1):27–34. doi: 10.1007/BF00224715. [DOI] [PubMed] [Google Scholar]
- Knowles D. M., 2nd, Tolidjian B., Marboe C., D'Agati V., Grimes M., Chess L. Monoclonal anti-human monocyte antibodies OKM1 and OKM5 possess distinctive tissue distributions including differential reactivity with vascular endothelium. J Immunol. 1984 May;132(5):2170–2173. [PubMed] [Google Scholar]
- Miossec P., Cavender D., Ziff M. Production of interleukin 1 by human endothelial cells. J Immunol. 1986 Apr 1;136(7):2486–2491. [PubMed] [Google Scholar]
- Nagura H., Koshikawa T., Fukuda Y., Asai J. Hepatic vascular endothelial cells heterogenously express surface antigens associated with monocytes, macrophages and T lymphocytes. Virchows Arch A Pathol Anat Histopathol. 1986;409(4):407–416. doi: 10.1007/BF00705413. [DOI] [PubMed] [Google Scholar]
- Nunez G., Ball E. J., Stastny P. Accessory cell function of human endothelial cells. I. A subpopulation of Ia positive cells is required for antigen presentation. J Immunol. 1983 Aug;131(2):666–673. [PubMed] [Google Scholar]
- Osborn L., Hession C., Tizard R., Vassallo C., Luhowskyj S., Chi-Rosso G., Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989 Dec 22;59(6):1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- Parums D. V., Cordell J. L., Micklem K., Heryet A. R., Gatter K. C., Mason D. Y. JC70: a new monoclonal antibody that detects vascular endothelium associated antigen on routinely processed tissue sections. J Clin Pathol. 1990 Sep;43(9):752–757. doi: 10.1136/jcp.43.9.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul L. C., Baldwin W. M., Van Es L. A. Transplantation antigens on renal endothelium. Neth J Med. 1982;25(7):208–214. [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr Expression of Ia-like antigens by human vascular endothelial cells is inducible in vitro: demonstration by monoclonal antibody binding and immunoprecipitation. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6641–6645. doi: 10.1073/pnas.79.21.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerance A., Stovin P. G. Heart transplant pathology: the British experience. J Clin Pathol. 1985 Feb;38(2):146–159. doi: 10.1136/jcp.38.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. L., Page C., Hengstenberg C., Yacoub M. H. Identification of antigen presenting cells in normal and transplanted human heart: importance of endothelial cells. Hum Immunol. 1990 Jun;28(2):179–185. doi: 10.1016/0198-8859(90)90017-j. [DOI] [PubMed] [Google Scholar]
- Ryan U. S., Schultz D. R., Ruan J. W. Fc and C3b receptors on pulmonary endothelial cells: induction by injury. Science. 1981 Oct 30;214(4520):557–558. doi: 10.1126/science.6270789. [DOI] [PubMed] [Google Scholar]
- Salomon R. N., Hughes C. C., Schoen F. J., Payne D. D., Pober J. S., Libby P. Human coronary transplantation-associated arteriosclerosis. Evidence for a chronic immune reaction to activated graft endothelial cells. Am J Pathol. 1991 Apr;138(4):791–798. [PMC free article] [PubMed] [Google Scholar]
- Schlingemann R. O., Dingjan G. M., Emeis J. J., Blok J., Warnaar S. O., Ruiter D. J. Monoclonal antibody PAL-E specific for endothelium. Lab Invest. 1985 Jan;52(1):71–76. [PubMed] [Google Scholar]
- Shen H. H., Talle M. A., Goldstein G., Chess L. Functional subsets of human monocytes defined by monoclonal antibodies: a distinct subset of monocytes contains the cells capable of inducing the autologous mixed lymphocyte culture. J Immunol. 1983 Feb;130(2):698–705. [PubMed] [Google Scholar]
- Sironi M., Breviario F., Proserpio P., Biondi A., Vecchi A., Van Damme J., Dejana E., Mantovani A. IL-1 stimulates IL-6 production in endothelial cells. J Immunol. 1989 Jan 15;142(2):549–553. [PubMed] [Google Scholar]
- Stockinger H., Gadd S. J., Eher R., Majdic O., Schreiber W., Kasinrerk W., Strass B., Schnabl E., Knapp W. Molecular characterization and functional analysis of the leukocyte surface protein CD31. J Immunol. 1990 Dec 1;145(11):3889–3897. [PubMed] [Google Scholar]
- Taylor P. M., Rose M. L., Yacoub M. H. Expression of MHC antigens in normal human lungs and transplanted lungs with obliterative bronchiolitis. Transplantation. 1989 Sep;48(3):506–510. doi: 10.1097/00007890-198909000-00030. [DOI] [PubMed] [Google Scholar]
- Turner R. R., Beckstead J. H., Warnke R. A., Wood G. S. Endothelial cell phenotypic diversity. In situ demonstration of immunologic and enzymatic heterogeneity that correlates with specific morphologic subtypes. Am J Clin Pathol. 1987 May;87(5):569–575. doi: 10.1093/ajcp/87.5.569. [DOI] [PubMed] [Google Scholar]
- Van Epps D. E., Potter J., Vachula M., Smith C. W., Anderson D. C. Suppression of human lymphocyte chemotaxis and transendothelial migration by anti-LFA-1 antibody. J Immunol. 1989 Nov 15;143(10):3207–3210. [PubMed] [Google Scholar]
- Wagner C. R., Vetto R. M., Burger D. R. Subcultured human endothelial cells can function independently as fully competent antigen-presenting cells. Hum Immunol. 1985 May;13(1):33–47. doi: 10.1016/0198-8859(85)90025-4. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Shimokata K., Nagura H. An immunohistochemical study on phenotypic heterogeneity of human pulmonary vascular endothelial cells. Virchows Arch A Pathol Anat Histopathol. 1988;412(5):479–486. doi: 10.1007/BF00750582. [DOI] [PubMed] [Google Scholar]