Abstract
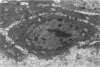
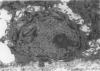
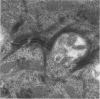
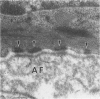
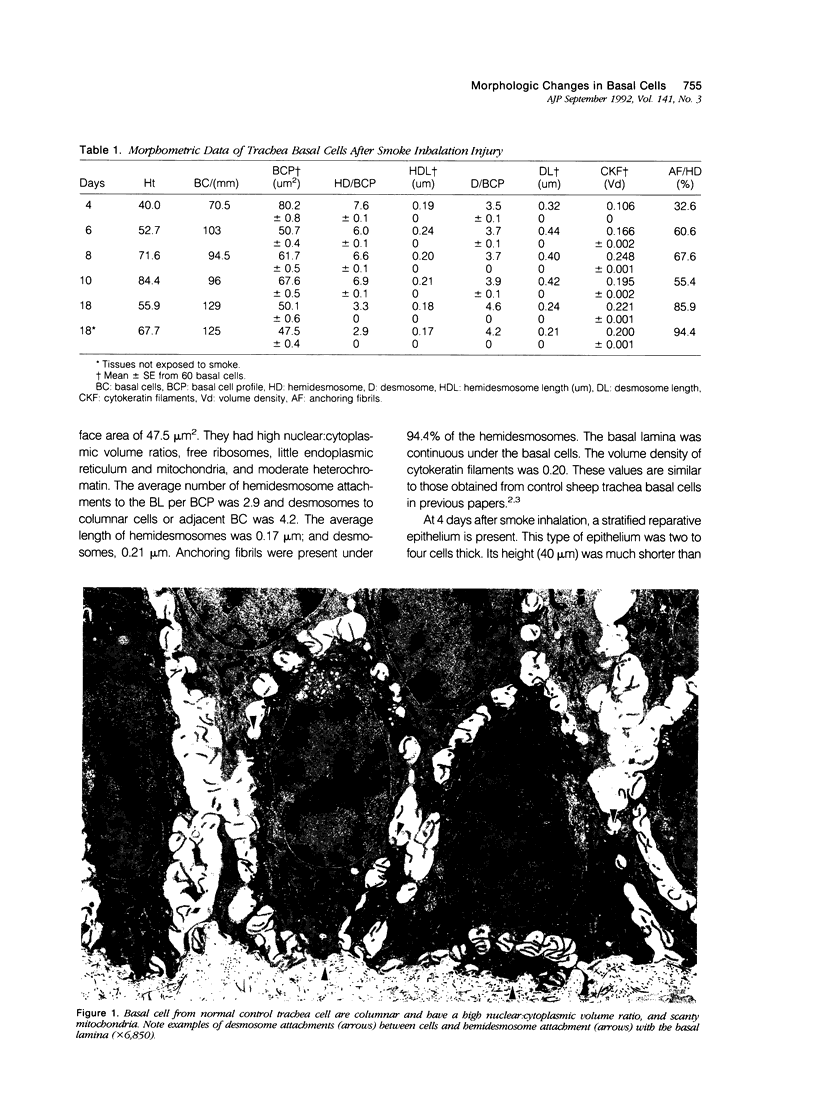
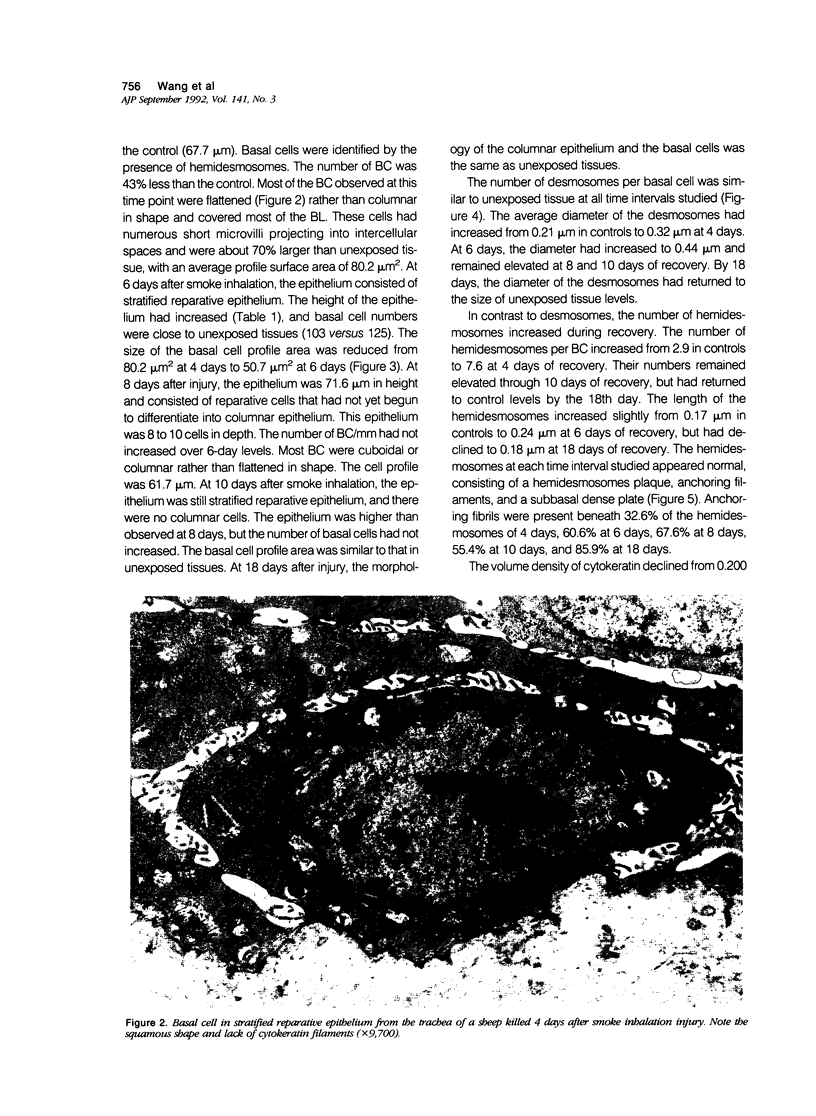
Basal cells are differentiated with respect to junctional adhesion mechanisms and play a role in attachment of columnar epithelium to the basal lamina. Although much is known about nonciliated and ciliated cell differentiation during the repair process after injury, little is known about the basal cell. We studied the morphology of basal cells and quantitated junctional adhesion structures during repair of tracheal epithelium exposed to toxic cotton smoke. Ten adult ewes were given a smoke injury to a portion of the upper cervical trachea and were killed at 4, 6, 8, 10, and 18 days after injury for morphometric studies. At 4 days, there was a stratified reparative epithelium over the basal lamina, which was two to four cells in depth. The basal cells were identified by their hemidesmosome (HD) attachment to the basal lamina. Basal cells were about 69% larger than controls and flattened rather than columnar. The amount of HD attachment was 192% greater than controls. In contrast, volume density of cytokeratin filaments had decreased about 47%. Basal cells had returned to normal numbers and size and a columnar shape by day 18. The amount of desmosome (D) and HD attachment and volume density of cytokeratins had also reached control levels by day 18. These data indicate that morphology of basal cells changes during the initial stages of reparative regeneration but returns to normal by 18 days. Morphologic changes appear to reflect changes in size of the cell associated with cell division rather than differentiation of recently divided basal cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrow R. E., Morris S. E., Basadre J. O., Herndon D. N. Selective permeability changes in the lungs and airways of sheep after toxic smoke inhalation. J Appl Physiol (1985) 1990 May;68(5):2165–2170. doi: 10.1152/jappl.1990.68.5.2165. [DOI] [PubMed] [Google Scholar]
- Barrow R. E., Morris S. E., Linares H. A., Herndon D. N. Tracheal venous blood and lymph collection: a model to study airway injury in sheep. J Appl Physiol (1985) 1991 Apr;70(4):1645–1649. doi: 10.1152/jappl.1991.70.4.1645. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Cox R. A., Burke A. S., Moller P. C. Differentiation of anchoring junctions in tracheal basal cells in the growing rat. Am J Respir Cell Mol Biol. 1992 Feb;6(2):153–157. doi: 10.1165/ajrcmb/6.2.153. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Cox R. A., Shami S. G., Plopper C. G. Junctional adhesion mechanisms in airway basal cells. Am J Respir Cell Mol Biol. 1990 Oct;3(4):341–347. doi: 10.1165/ajrcmb/3.4.341. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Cox R. A., Shami S. G., Wilson B., Plopper C. G. The role of basal cells in attachment of columnar cells to the basal lamina of the trachea. Am J Respir Cell Mol Biol. 1989 Dec;1(6):463–469. doi: 10.1165/ajrcmb/1.6.463. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Moller P. C. Biology of airway basal cells. Exp Lung Res. 1991 May-Jun;17(3):513–531. doi: 10.3109/01902149109062862. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Plopper C. G. The role of basal cells in adhesion of columnar epithelium to airway basement membrane. Am Rev Respir Dis. 1988 Aug;138(2):481–483. doi: 10.1164/ajrccm/138.2.481. [DOI] [PubMed] [Google Scholar]
- Garrod D. R. Desmosomes, cell adhesion molecules and the adhesive properties of cells in tissues. J Cell Sci Suppl. 1986;4:221–237. doi: 10.1242/jcs.1986.supplement_4.14. [DOI] [PubMed] [Google Scholar]
- Gipson I. K., Spurr-Michaud S. J., Tisdale A. S. Hemidesmosomes and anchoring fibril collagen appear synchronously during development and wound healing. Dev Biol. 1988 Apr;126(2):253–262. doi: 10.1016/0012-1606(88)90136-4. [DOI] [PubMed] [Google Scholar]
- Gipson I. K., Spurr-Michaud S., Tisdale A., Keough M. Reassembly of the anchoring structures of the corneal epithelium during wound repair in the rabbit. Invest Ophthalmol Vis Sci. 1989 Mar;30(3):425–434. [PubMed] [Google Scholar]
- Kauffman S. L. Cell proliferation in the mammalian lung. Int Rev Exp Pathol. 1980;22:131–191. [PubMed] [Google Scholar]
- Krawczyk W. S., Wilgram G. F. Hemidesmosome and desmosome morphogenesis during epidermal wound healing. J Ultrastruct Res. 1973 Oct;45(1):93–101. doi: 10.1016/s0022-5320(73)90035-x. [DOI] [PubMed] [Google Scholar]
- Kurpakus M. A., Jones J. C. A novel hemidesmosomal plaque component: tissue distribution and incorporation into assembling hemidesmosomes in an in vitro model. Exp Cell Res. 1991 May;194(1):139–146. doi: 10.1016/0014-4827(91)90143-i. [DOI] [PubMed] [Google Scholar]
- Kurpakus M. A., Stock E. L., Jones J. C. Analysis of wound healing in an in vitro model: early appearance of laminin and a 125 x 10(3) Mr polypeptide during adhesion complex formation. J Cell Sci. 1990 Aug;96(Pt 4):651–660. doi: 10.1242/jcs.96.4.651. [DOI] [PubMed] [Google Scholar]
- Moll R., Cowin P., Kapprell H. P., Franke W. W. Desmosomal proteins: new markers for identification and classification of tumors. Lab Invest. 1986 Jan;54(1):4–25. [PubMed] [Google Scholar]
- Schwarz M. A., Owaribe K., Kartenbeck J., Franke W. W. Desmosomes and hemidesmosomes: constitutive molecular components. Annu Rev Cell Biol. 1990;6:461–491. doi: 10.1146/annurev.cb.06.110190.002333. [DOI] [PubMed] [Google Scholar]
- White F. H., Gohari K. Desmosomes in hamster cheek pouch epithelium: their quantitative characterization during epithelial differentiation. J Cell Sci. 1984 Mar;66:411–429. doi: 10.1242/jcs.66.1.411. [DOI] [PubMed] [Google Scholar]
- White F. H., Mayhew T. M., Gohari K. Stereological methods for quantifying cell surface specializations in epithelia, including a concept for counting desmosomes and hemidesmosomes. Br J Dermatol. 1982 Oct;107(4):401–414. doi: 10.1111/j.1365-2133.1982.tb00383.x. [DOI] [PubMed] [Google Scholar]