Abstract
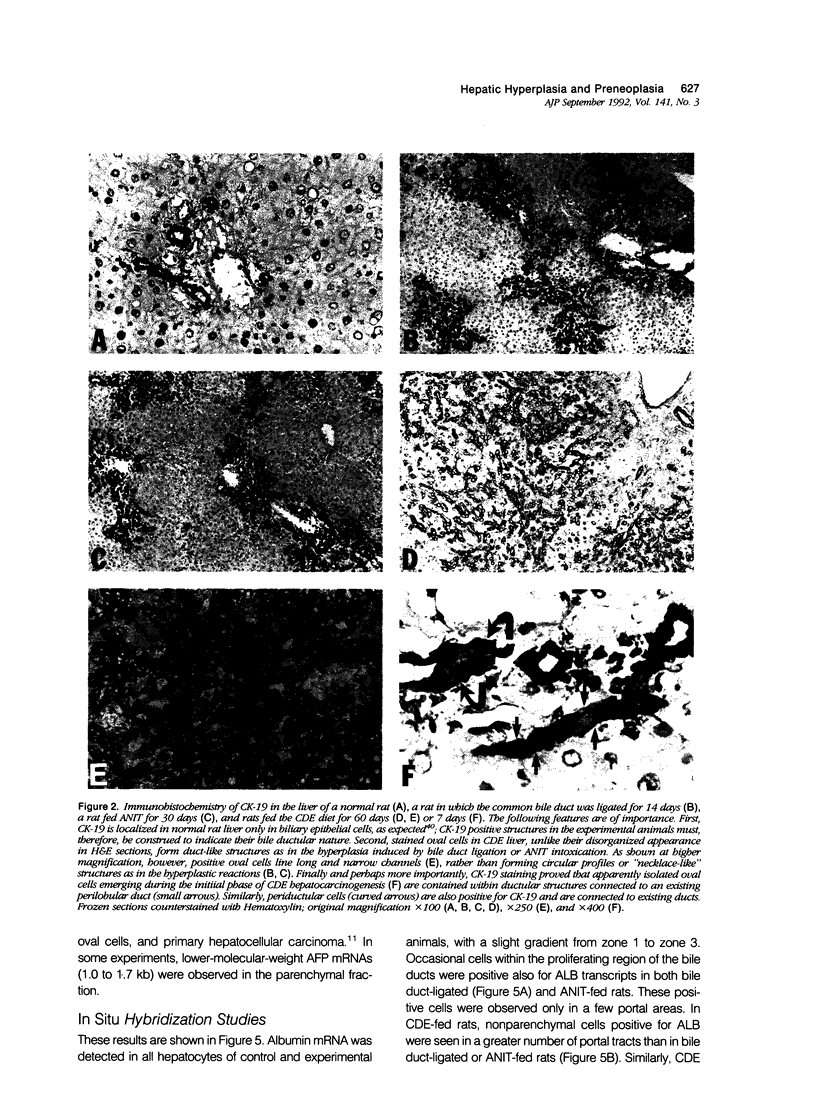
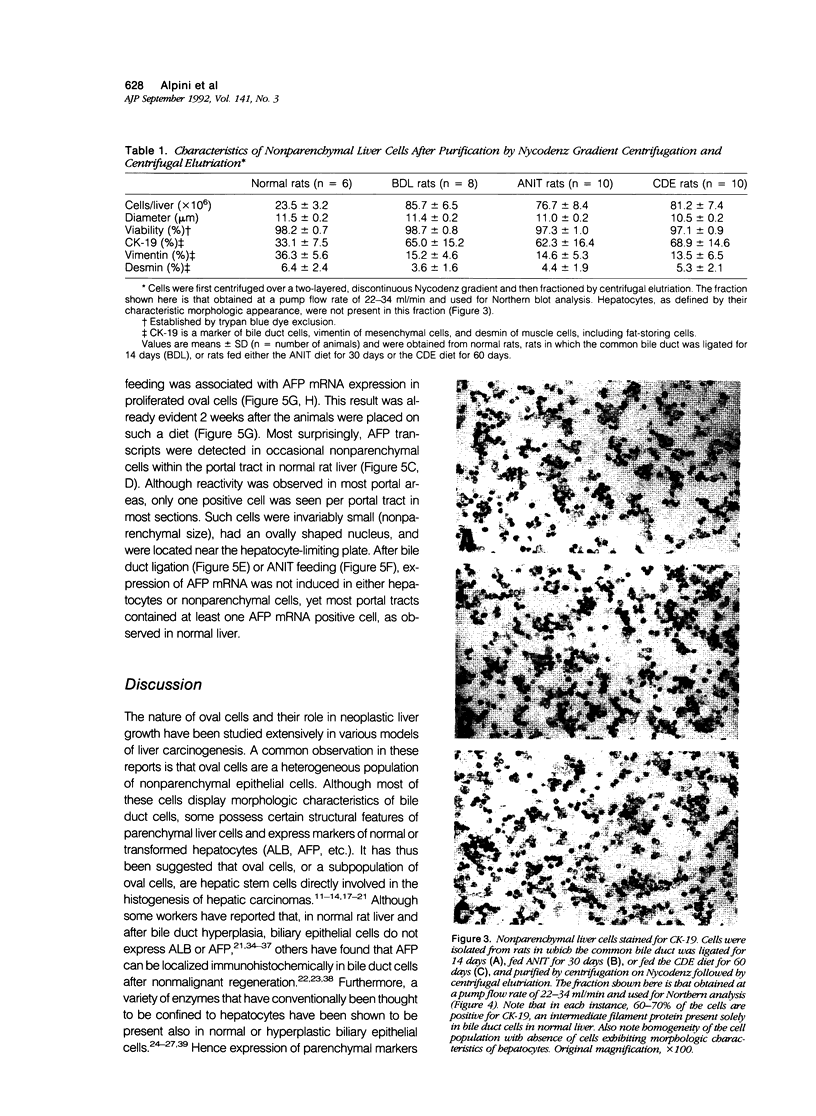
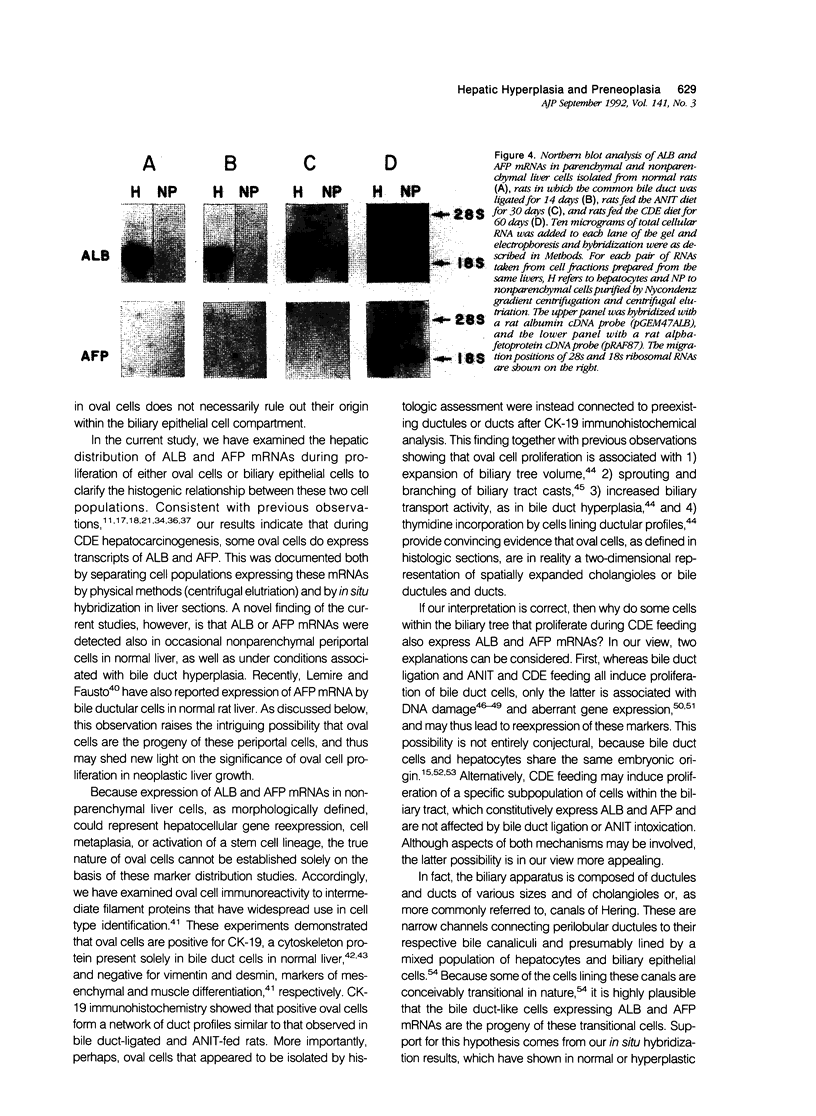
The nature of bile duct-like (oval) cells proliferating during chemical hepatocarcinogenesis has been controversial. To investigate this issue further, the authors compared the hepatic distribution of albumin (ALB) and alpha-fetoprotein (AFP) mRNAs in rats in which oval cell proliferation was induced by feeding a choline-devoid diet containing 0.1% ethionine (CDE, a hepatocarcinogenic diet) with that in normal rats and in rats in which biliary epithelial cell hyperplasia was induced by either bile duct ligation or feeding alpha-naphthylisothiocyanate (ANIT). Northern blot analysis in parenchymal and nonparenchymal liver cells isolated from these animals demonstrated that ALB mRNA was present in the hepatocytes of both control and experimental animals, whereas this transcript was detected in nonparenchymal epithelial cells only in CDE-fed rats. Alpha-fetoprotein mRNA was not seen in either parenchymal or nonparenchymal cells isolated from normal or hyperplastic livers induced by bile duct ligation or ANIT feeding. In CDE-fed rats, however, both parenchymal and nonparenchymal cell populations displayed AFP message. In situ hybridization directly demonstrated nonparenchymal cell expression of both ALB and AFP transcripts in CDE-fed rats. Most surprisingly, ALB and AFP mRNAs were also detected by in situ hybridization in occasional nonparenchymal cells located in portal tracts near the limiting plate in normal liver, as well as under conditions associated with bile duct hyperplasia. Immunohistochemical studies of intermediate filament proteins, cytokeratin 19 (a marker of glandular epithelia), vimentin (a marker of mesenchymal lineage), and desmin (a marker of muscle cell differentiation) demonstrated that oval cells, as well as normal and hyperplastic bile duct cells, were positive for cytokeratin 19 and negative for both vimentin and desmin. Cytokeratin-positive oval cells formed duct profiles and were connected to preexisting ductules and ducts. These results are construed to suggest that oval cells proliferating during CDE hepatocarcinogenesis are derived from epithelial cells within the biliary tree. The presence of cells with similar morphologic appearance, periportal location, and AFP and ALB expression in normal liver suggests that these cells may be the progenitors of oval cells induced by some carcinogenic regimens.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpini G., Lenzi R., Sarkozi L., Tavoloni N. Biliary physiology in rats with bile ductular cell hyperplasia. Evidence for a secretory function of proliferated bile ductules. J Clin Invest. 1988 Feb;81(2):569–578. doi: 10.1172/JCI113355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpini G., Lenzi R., Zhai W. R., Liu M. H., Slott P. A., Paronetto F., Tavoloni N. Isolation of a nonparenchymal liver cell fraction enriched in cells with biliary epithelial phenotypes. Gastroenterology. 1989 Nov;97(5):1248–1260. doi: 10.1016/0016-5085(89)91696-x. [DOI] [PubMed] [Google Scholar]
- Alpini G., Lenzi R., Zhai W. R., Slott P. A., Liu M. H., Sarkozi L., Tavoloni N. Bile secretory function of intrahepatic biliary epithelium in the rat. Am J Physiol. 1989 Jul;257(1 Pt 1):G124–G133. doi: 10.1152/ajpgi.1989.257.1.G124. [DOI] [PubMed] [Google Scholar]
- Bannasch P., Zerban H. Pathogenesis of primary liver tumors induced by chemicals. Recent Results Cancer Res. 1986;100:1–15. doi: 10.1007/978-3-642-82635-1_1. [DOI] [PubMed] [Google Scholar]
- Benner U., Hacker H. J., Bannasch P. Electron microscopical demonstration of glucose-6-phosphatase in native cryostat sections fixed with glutaraldehyde through semipermeable membranes. Histochemistry. 1979;65(1):41–47. doi: 10.1007/BF00496684. [DOI] [PubMed] [Google Scholar]
- Braun L., Goyette M., Yaswen P., Thompson N. L., Fausto N. Growth in culture and tumorigenicity after transfection with the ras oncogene of liver epithelial cells from carcinogen-treated rats. Cancer Res. 1987 Aug 1;47(15):4116–4124. [PubMed] [Google Scholar]
- Braun L., Mikumo R., Fausto N. Production of hepatocellular carcinoma by oval cells: cell cycle expression of c-myc and p53 at different stages of oval cell transformation. Cancer Res. 1989 Mar 15;49(6):1554–1561. [PubMed] [Google Scholar]
- Cameron R. G. Comparison of GST-P versus GGT as markers of hepatocellular lineage during analyses of initiation of carcinogenesis. Cancer Invest. 1988;6(6):725–734. doi: 10.3109/07357908809078039. [DOI] [PubMed] [Google Scholar]
- Chandar N., Lombardi B., Locker J. c-myc gene amplification during hepatocarcinogenesis by a choline-devoid diet. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2703–2707. doi: 10.1073/pnas.86.8.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- De Lü M., Miyazaki K., Yoshitomi S., Nakayama F. DNA repair synthesis in primary culture of bovine bile duct epithelial cells induced by chemical agents in relation to bile duct cancer. Mutat Res. 1988 Jul;194(1):73–79. doi: 10.1016/0167-8817(88)90058-2. [DOI] [PubMed] [Google Scholar]
- Evarts R. P., Nagy P., Marsden E., Thorgeirsson S. S. A precursor-product relationship exists between oval cells and hepatocytes in rat liver. Carcinogenesis. 1987 Nov;8(11):1737–1740. doi: 10.1093/carcin/8.11.1737. [DOI] [PubMed] [Google Scholar]
- Evarts R. P., Nagy P., Marsden E., Thorgeirsson S. S. In situ hybridization studies on expression of albumin and alpha-fetoprotein during the early stage of neoplastic transformation in rat liver. Cancer Res. 1987 Oct 15;47(20):5469–5475. [PubMed] [Google Scholar]
- Evarts R. P., Nagy P., Nakatsukasa H., Marsden E., Thorgeirsson S. S. In vivo differentiation of rat liver oval cells into hepatocytes. Cancer Res. 1989 Mar 15;49(6):1541–1547. [PubMed] [Google Scholar]
- Evarts R. P., Nakatsukasa H., Marsden E. R., Hsia C. C., Dunsford H. A., Thorgeirsson S. S. Cellular and molecular changes in the early stages of chemical hepatocarcinogenesis in the rat. Cancer Res. 1990 Jun 1;50(11):3439–3444. [PubMed] [Google Scholar]
- FARBER E. Similarities in the sequence of early histological changes induced in the liver of the rat by ethionine, 2-acetylamino-fluorene, and 3'-methyl-4-dimethylaminoazobenzene. Cancer Res. 1956 Feb;16(2):142–148. [PubMed] [Google Scholar]
- Farber E., Sarma D. S. Chemical carcinogenesis: the liver as a model. Pathol Immunopathol Res. 1986;5(1):1–28. doi: 10.1159/000157000. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Denk H., Kalt R., Schmid E. Biochemical and immunological identification of cytokeratin proteins present in hepatocytes of mammalian liver tissue. Exp Cell Res. 1981 Feb;131(2):299–318. doi: 10.1016/0014-4827(81)90234-2. [DOI] [PubMed] [Google Scholar]
- Franson K. L., Burchell B., Mathis G. A. 17 Beta-hydroxysteroid UDP-glucuronosyl transferase is expressed in bile ductular epithelial cells under physiological conditions. Biochem Biophys Res Commun. 1990 Dec 31;173(3):1001–1007. doi: 10.1016/s0006-291x(05)80885-x. [DOI] [PubMed] [Google Scholar]
- GOLDFARB S., SINGER E. J., POPPER H. Experimental cholangitis due to alpha-naphthyl-isothiocyanate (ANIT). Am J Pathol. 1962 Jun;40:685–698. [PMC free article] [PubMed] [Google Scholar]
- GRISHAM J. W., HARTROFT W. S. Morphologic identification by electron microscopy of "oval" cells in experimental hepatic degeneration. Lab Invest. 1961 Mar-Apr;10:317–332. [PubMed] [Google Scholar]
- Goldfarb S. A morphological and histochemical study of carcinogenesis of the liver in rats fed 3'-methyl-4-dimethylaminoazobenzene. Cancer Res. 1973 May;33(5):1119–1128. [PubMed] [Google Scholar]
- Jagodzinski L. L., Sargent T. D., Yang M., Glackin C., Bonner J. Sequence homology between RNAs encoding rat alpha-fetoprotein and rat serum albumin. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3521–3525. doi: 10.1073/pnas.78.6.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp S. A., Green M. D., Tephly T. R., Baron J. Immunohistochemical demonstration of isozyme- and strain-specific differences in the intralobular localizations and distributions of UDP-glucuronosyltransferases in livers of untreated rats. Mol Pharmacol. 1988 Jan;33(1):14–21. [PubMed] [Google Scholar]
- Koen H., Pugh T. D., Nychka D., Goldfarb S. Presence of alpha-fetoprotein-positive cells in hepatocellular foci and microcarcinomas induced by single injections of diethylnitrosamine in infant mice. Cancer Res. 1983 Feb;43(2):702–708. [PubMed] [Google Scholar]
- Kuhlmann W. D., Wurster K. Correlation of histology and alpha 1-fetoprotein resurgence in rat liver regeneration after experimental injury by galactosamine. Virchows Arch A Pathol Anat Histol. 1980;387(1):47–57. doi: 10.1007/BF00428428. [DOI] [PubMed] [Google Scholar]
- Lemire J. M., Fausto N. Multiple alpha-fetoprotein RNAs in adult rat liver: cell type-specific expression and differential regulation. Cancer Res. 1991 Sep 1;51(17):4656–4664. [PubMed] [Google Scholar]
- Lenzi R., Liu M. H., Lenzen R., Han T., Alpini G., Tavoloni N. Distribution of glucose-6-phosphatase activity in normal, hyperplastic, and preneoplastic rat liver. Virchows Arch B Cell Pathol Incl Mol Pathol. 1991;61(4):279–287. doi: 10.1007/BF02890429. [DOI] [PubMed] [Google Scholar]
- Lenzi R., Liu M. H., Tarsetti F., Slott P. A., Alpini G., Zhai W. R., Paronetto F., Lenzen R., Tavoloni N. Histogenesis of bile duct-like cells proliferating during ethionine hepatocarcinogenesis. Evidence for a biliary epithelial nature of oval cells. Lab Invest. 1992 Mar;66(3):390–402. [PubMed] [Google Scholar]
- Li D. H., Xu D. C., Chandar N., Lombardi B., Randerath K. Persistent reduction of indigenous DNA modification (I-compound) levels in liver DNA from male Fischer rats fed choline-devoid diet and in DNA of resulting neoplasms. Cancer Res. 1990 Dec 1;50(23):7577–7580. [PubMed] [Google Scholar]
- Makino Y., Yamamoto K., Tsuji T. Three-dimensional arrangement of ductular structures formed by oval cells during hepatocarcinogenesis. Acta Med Okayama. 1988 Jun;42(3):143–150. doi: 10.18926/AMO/31029. [DOI] [PubMed] [Google Scholar]
- Marceau N., Blouin M. J., Germain L., Noel M. Role of different epithelial cell types in liver ontogenesis, regeneration and neoplasia. In Vitro Cell Dev Biol. 1989 Apr;25(4):336–341. doi: 10.1007/BF02624596. [DOI] [PubMed] [Google Scholar]
- Marceau N. Cell lineages and differentiation programs in epidermal, urothelial and hepatic tissues and their neoplasms. Lab Invest. 1990 Jul;63(1):4–20. [PubMed] [Google Scholar]
- Mathis G. A., Walls S. A., D'Amico P., Gengo T. F., Sirica A. E. Enzyme profile of rat bile ductular epithelial cells in reference to the resistance phenotype in hepatocarcinogenesis. Hepatology. 1989 Mar;9(3):477–485. doi: 10.1002/hep.1840090323. [DOI] [PubMed] [Google Scholar]
- Ogawa K., Minase T., Onhoe T. Demonstration of glucose 6-phosphatase activity in the oval cells of rat liver and the significance of the oval cells in azo dye carcinogenesis. Cancer Res. 1974 Dec;34(12):3379–3386. [PubMed] [Google Scholar]
- Osborn M., Weber K. Intermediate filaments: cell-type-specific markers in differentiation and pathology. Cell. 1982 Dec;31(2 Pt 1):303–306. doi: 10.1016/0092-8674(82)90122-2. [DOI] [PubMed] [Google Scholar]
- POPPER H., KENT G., STEIN R. Ductular cell reaction in the liver in hepatic injury. J Mt Sinai Hosp N Y. 1957 Sep-Oct;24(5):551–556. [PubMed] [Google Scholar]
- PRICE J. M., HARMAN J. W., MILLER E. C., MILLER J. A. Progressive microscopic alterations in the livers of rats fed the hepatic carcinogens 3'-methyl-4-dimethylaminoazobenzene and 4'-fluoro-4-dimethylaminoazobenzene. Cancer Res. 1952 Mar;12(3):192–200. [PubMed] [Google Scholar]
- Petropoulos C. J., Yaswen P., Panzica M., Fausto N. Cell lineages in liver carcinogenesis: possible clues from studies of the distribution of alpha-fetoprotein RNA sequences in cell populations isolated from normal, regenerating, and preneoplastic rat livers. Cancer Res. 1985 Nov;45(11 Pt 2):5762–5768. [PubMed] [Google Scholar]
- Richards W. L., Tsukada Y., Potter V. R. gamma-Glutamyl transpeptidase and alpha-fetoprotein expression during alpha-naphthylisothiocyanate-induced hepatotoxicity in rats. Cancer Res. 1982 Dec;42(12):5133–5138. [PubMed] [Google Scholar]
- Rushmore T. H., Farber E., Ghoshal A. K., Parodi S., Pala M., Taningher M. A choline-devoid diet, carcinogenic in the rat, induces DNA damage and repair. Carcinogenesis. 1986 Oct;7(10):1677–1680. doi: 10.1093/carcin/7.10.1677. [DOI] [PubMed] [Google Scholar]
- Scoazec J. Y., Moreau A., Feldmann G., Bernuau D. Cellular expression of alpha-fetoprotein gene and its relation to albumin gene expression during rat azo-dye hepatocarcinogenesis. Cancer Res. 1989 Apr 1;49(7):1790–1796. [PubMed] [Google Scholar]
- Sell S., Dunsford H. A. Evidence for the stem cell origin of hepatocellular carcinoma and cholangiocarcinoma. Am J Pathol. 1989 Jun;134(6):1347–1363. [PMC free article] [PubMed] [Google Scholar]
- Shiojiri N. The origin of intrahepatic bile duct cells in the mouse. J Embryol Exp Morphol. 1984 Feb;79:25–39. [PubMed] [Google Scholar]
- Shivapurkar N., Wilson M. J., Poirier L. A. Hypomethylation of DNA in ethionine-fed rats. Carcinogenesis. 1984 Aug;5(8):989–992. doi: 10.1093/carcin/5.8.989. [DOI] [PubMed] [Google Scholar]
- Slott P. A., Liu M. H., Tavoloni N. Origin, pattern, and mechanism of bile duct proliferation following biliary obstruction in the rat. Gastroenterology. 1990 Aug;99(2):466–477. doi: 10.1016/0016-5085(90)91030-a. [DOI] [PubMed] [Google Scholar]
- Steiner J. W., Carruthers J. S. Studies on the Fine Structure of the Terminal Branches of the Biliary Tree: I. The Morphology of Normal Bile Canaliculi, Bile Pre-ductules (Ducts of Hering) and Bile Ductules. Am J Pathol. 1961 Jun;38(6):639–661. [PMC free article] [PubMed] [Google Scholar]
- Tournier I., Legrès L., Schoevaert D., Feldmann G., Bernuau D. Cellular analysis of alpha-fetoprotein gene activation during carbon tetrachloride and D-galactosamine-induced acute liver injury in rats. Lab Invest. 1988 Nov;59(5):657–665. [PubMed] [Google Scholar]
- Van Eyken P., Sciot R., Desmet V. Intrahepatic bile duct development in the rat: a cytokeratin-immunohistochemical study. Lab Invest. 1988 Jul;59(1):52–59. [PubMed] [Google Scholar]
- Yaswen P., Goyette M., Shank P. R., Fausto N. Expression of c-Ki-ras, c-Ha-ras, and c-myc in specific cell types during hepatocarcinogenesis. Mol Cell Biol. 1985 Apr;5(4):780–786. doi: 10.1128/mcb.5.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama S., Satoh M., Lombardi B. Bile ductular cells and the phenotypic heterogeneity of the population of hepatic non-parenchymal epithelial cells induced in rats by chemical carcinogens. Carcinogenesis. 1986 Jul;7(7):1215–1219. doi: 10.1093/carcin/7.7.1215. [DOI] [PubMed] [Google Scholar]
- Yoshimura H., Harris R., Yokoyama S., Takahashi S., Sells M. A., Pan S. F., Lombardi B. Anaplastic carcinomas in nude mice and in original donor strain rats inoculated with cultured oval cells. Am J Pathol. 1983 Mar;110(3):322–332. [PMC free article] [PubMed] [Google Scholar]
- van Eyken P., Sciot R., van Damme B., de Wolf-Peeters C., Desmet V. J. Keratin immunohistochemistry in normal human liver. Cytokeratin pattern of hepatocytes, bile ducts and acinar gradient. Virchows Arch A Pathol Anat Histopathol. 1987;412(1):63–72. doi: 10.1007/BF00750732. [DOI] [PubMed] [Google Scholar]