Abstract
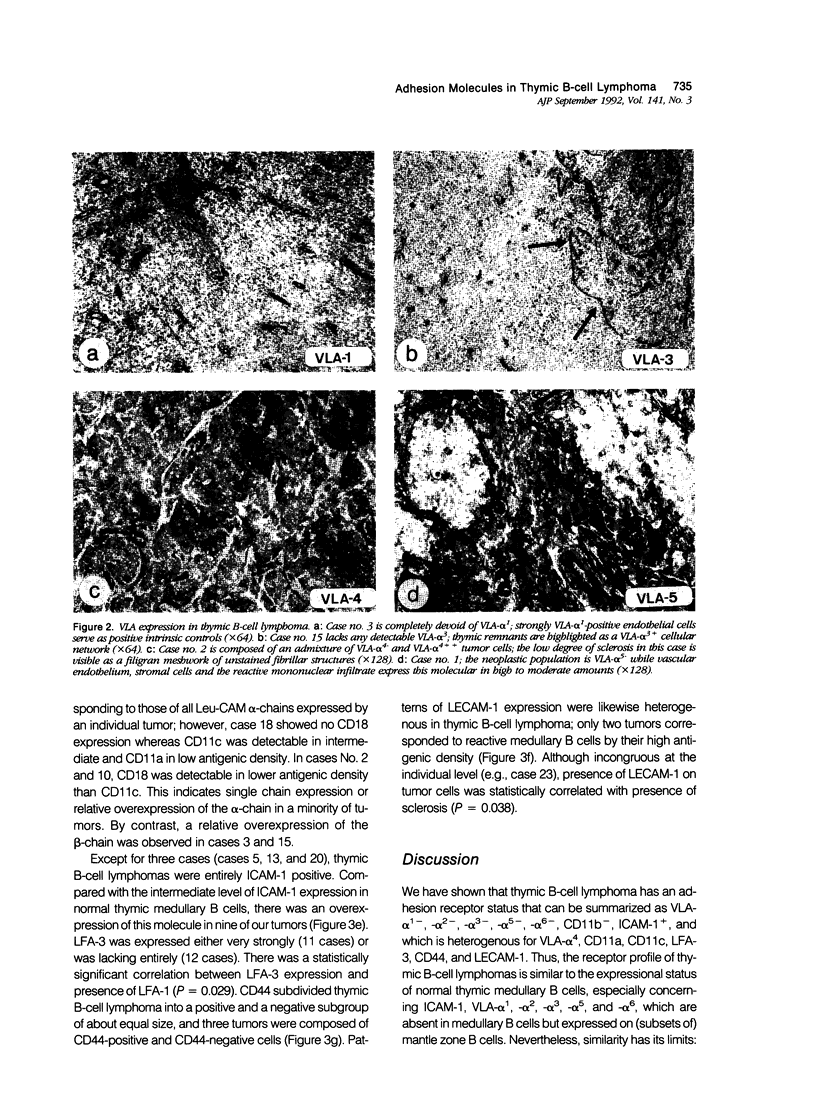
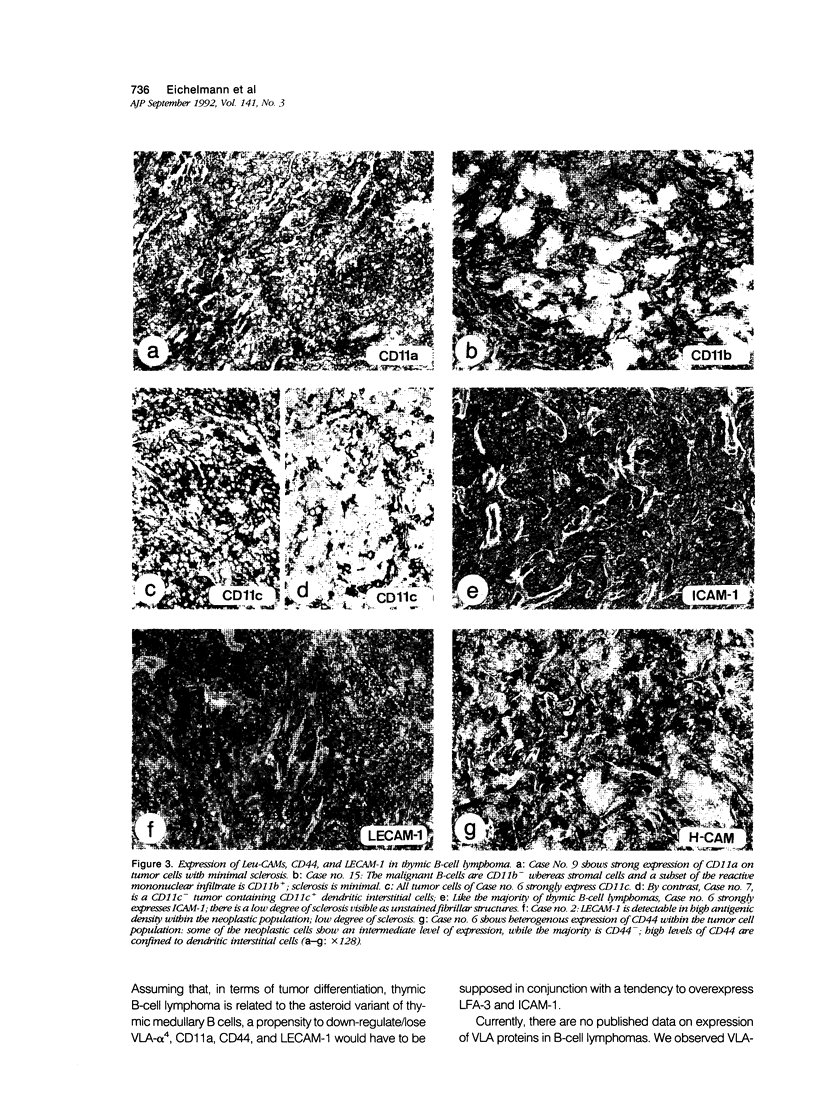
Primary thymic B-cell lymphoma is clinically characterized by aleukemic, highly aggressive local growth, infrequent distant metastasis, and infrequent secondary lymph node involvement. VLA-1 to VLA-6 are cell surface molecules binding to matrix molecules such as collagen, fibronectin, epiligrin, and laminin. VLA-4 additionally binds to VCAM-1 and ICAM-2, thus mediating intercellular adhesion. Other molecules involved in cell/cell adhesion are LFA-1 (CD11a/CD18), Mac-1(CD11b/CD18) and their ligand ICAM-1 (CD54), p150,95 (CD11c/CD18), LFA-3 (CD58), CD44, and LECAM-1. Twenty-three tumors, together with normal lymphoid tissue, were immunohistochemically examined to investigate the expression pattern of these molecules in thymic B-cell lymphomas and in their putative normal counterparts, namely thymic medullary B cells. Thymic B-cell lymphomas consistently lacked VLA-1,-2,-3,-5,-6, and CD11b, expressed ICAM-1 in 21 of 23 cases but were heterogenous for VLA-4, LFA-1, CD11c, LFA-3, CD44, and LECAM-1. Presence of LFA-1 correlated with LFA-3 expression (P = 0.029). The receptor profile of thymic B-cell lymphoma was reminiscent of the expressional status of normal thymic medullary B cells in some aspects but deviated in others: Assuming that, in terms of differentiation, thymic B-cell lymphoma is related to the asteroid variant of thymic medullary B cells, a propensity to down-regulate/lose VLA-4, CD11a, CD44, and LECAM-1 would have to be supposed in conjunction with a tendency to overexpress ICAM-1 and LFA-3. Sclerosis as an inconsistent phenomenon in thymic B-cell lymphoma was absent in 8 of 23 tumors. Presence of sclerosis correlated with LECAM-1 expression of the tumor cells (P = 0.038). Recent studies suggest that a locally growing/aleukemic phenotype of a B-cell neoplasia might be determined by the phenotype VLAs-, LFA-1+, ICAM-1+, CD44-, and LECAM-1-. Our data corroborate this view.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addis B. J., Isaacson P. G. Large cell lymphoma of the mediastinum: a B-cell tumour of probable thymic origin. Histopathology. 1986 Apr;10(4):379–390. doi: 10.1111/j.1365-2559.1986.tb02491.x. [DOI] [PubMed] [Google Scholar]
- Berg E. L., Robinson M. K., Warnock R. A., Butcher E. C. The human peripheral lymph node vascular addressin is a ligand for LECAM-1, the peripheral lymph node homing receptor. J Cell Biol. 1991 Jul;114(2):343–349. doi: 10.1083/jcb.114.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billaud M., Rousset F., Calender A., Cordier M., Aubry J. P., Laisse V., Lenoir G. M. Low expression of lymphocyte function-associated antigen (LFA)-1 and LFA-3 adhesion molecules is a common trait in Burkitt's lymphoma associated with and not associated with Epstein-Barr virus. Blood. 1990 May 1;75(9):1827–1833. [PubMed] [Google Scholar]
- Boyd A. W., Dunn S. M., Fecondo J. V., Culvenor J. G., Dührsen U., Burns G. F., Wawryk S. O. Regulation of expression of a human intercellular adhesion molecule (ICAM-1) during lymphohematopoietic differentiation. Blood. 1989 May 15;73(7):1896–1903. [PubMed] [Google Scholar]
- Brandter L. B., Smith C. I., Hammarström L., Lindemalm C., Christensson B. Clonal immunoglobulin gene rearrangements in primary mediastinal clear cell lymphomas. Leukemia. 1989 Feb;3(2):122–129. [PubMed] [Google Scholar]
- Caligaris-Cappio F., Bergui L., Schena M., Chilosi M., Marchisio P. C. Cell-cell and cell-matrix adhesion structures may influence the growth pattern of chronic lymphoid malignancies. Leukemia. 1989 Mar;3(3):167–169. [PubMed] [Google Scholar]
- Carbone A., Manconi R., Poletti A., Gloghini A., De Paoli P., Volpe R. Expression of Leu-8 surface antigen in B-cell lymphomas. Correlation with other B-cell markers. J Pathol. 1988 Feb;154(2):133–140. doi: 10.1002/path.1711540205. [DOI] [PubMed] [Google Scholar]
- Clayberger C., Wright A., Medeiros L. J., Koller T. D., Link M. P., Smith S. D., Warnke R. A., Krensky A. M. Absence of cell surface LFA-1 as a mechanism of escape from immunosurveillance. Lancet. 1987 Sep 5;2(8558):533–536. doi: 10.1016/s0140-6736(87)92924-2. [DOI] [PubMed] [Google Scholar]
- Davis R. E., Dorfman R. F., Warnke R. A. Primary large-cell lymphoma of the thymus: a diffuse B-cell neoplasm presenting as primary mediastinal lymphoma. Hum Pathol. 1990 Dec;21(12):1262–1268. doi: 10.1016/s0046-8177(06)80040-7. [DOI] [PubMed] [Google Scholar]
- De la Hera A., Alvarez-Mon M., Sanchez-Madrid F., Martinez C., Durantez A. Co-expression of Mac-1 and p150,95 on CD5+ B cells. Structural and functional characterization in a human chronic lymphocytic leukemia. Eur J Immunol. 1988 Jul;18(7):1131–1134. doi: 10.1002/eji.1830180725. [DOI] [PubMed] [Google Scholar]
- Garcia-Pardo A., Wayner E. A., Carter W. G., Ferreira O. C., Jr Human B lymphocytes define an alternative mechanism of adhesion to fibronectin. The interaction of the alpha 4 beta 1 integrin with the LHGPEILDVPST sequence of the type III connecting segment is sufficient to promote cell attachment. J Immunol. 1990 May 1;144(9):3361–3366. [PubMed] [Google Scholar]
- Garcia C. F., Weiss L. M., Warnke R. A. Small noncleaved cell lymphoma: an immunophenotypic study of 18 cases and comparison with large cell lymphoma. Hum Pathol. 1986 May;17(5):454–461. doi: 10.1016/s0046-8177(86)80034-x. [DOI] [PubMed] [Google Scholar]
- Gregory C. D., Murray R. J., Edwards C. F., Rickinson A. B. Downregulation of cell adhesion molecules LFA-3 and ICAM-1 in Epstein-Barr virus-positive Burkitt's lymphoma underlies tumor cell escape from virus-specific T cell surveillance. J Exp Med. 1988 Jun 1;167(6):1811–1824. doi: 10.1084/jem.167.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemler M. E., Elices M. J., Parker C., Takada Y. Structure of the integrin VLA-4 and its cell-cell and cell-matrix adhesion functions. Immunol Rev. 1990 Apr;114:45–65. doi: 10.1111/j.1600-065x.1990.tb00561.x. [DOI] [PubMed] [Google Scholar]
- Hemler M. E. VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Annu Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- Hofmann W. J., Momburg F., Möller P., Otto H. F. Intra- and extrathymic B cells in physiologic and pathologic conditions. Immunohistochemical study on normal thymus and lymphofollicular hyperplasia of the thymus. Virchows Arch A Pathol Anat Histopathol. 1988;412(5):431–442. doi: 10.1007/BF00750577. [DOI] [PubMed] [Google Scholar]
- Hofmann W. J., Momburg F., Möller P. Thymic medullary cells expressing B lymphocyte antigens. Hum Pathol. 1988 Nov;19(11):1280–1287. doi: 10.1016/s0046-8177(88)80282-x. [DOI] [PubMed] [Google Scholar]
- Horst E., Meijer C. J., Radaskiewicz T., van Dongen J. J., Pieters R., Figdor C. G., Hooftman A., Pals S. T. Expression of a human homing receptor (CD44) in lymphoid malignancies and related stages of lymphoid development. Leukemia. 1990 May;4(5):383–389. [PubMed] [Google Scholar]
- Horst E., Meijer C. J., Radaszkiewicz T., Ossekoppele G. J., Van Krieken J. H., Pals S. T. Adhesion molecules in the prognosis of diffuse large-cell lymphoma: expression of a lymphocyte homing receptor (CD44), LFA-1 (CD11a/18), and ICAM-1 (CD54). Leukemia. 1990 Aug;4(8):595–599. [PubMed] [Google Scholar]
- Imai Y., True D. D., Singer M. S., Rosen S. D. Direct demonstration of the lectin activity of gp90MEL, a lymphocyte homing receptor. J Cell Biol. 1990 Sep;111(3):1225–1232. doi: 10.1083/jcb.111.3.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inghirami G., Wieczorek R., Zhu B. Y., Silber R., Dalla-Favera R., Knowles D. M. Differential expression of LFA-1 molecules in non-Hodgkin's lymphoma and lymphoid leukemia. Blood. 1988 Oct;72(4):1431–1434. [PubMed] [Google Scholar]
- Jacobson J. O., Aisenberg A. C., Lamarre L., Willett C. G., Linggood R. M., Miketic L. M., Harris N. L. Mediastinal large cell lymphoma. An uncommon subset of adult lymphoma curable with combined modality therapy. Cancer. 1988 Nov 1;62(9):1893–1898. doi: 10.1002/1097-0142(19881101)62:9<1893::aid-cncr2820620904>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Jalkanen S., Joensuu H., Klemi P. Prognostic value of lymphocyte homing receptor and S phase fraction in non-Hodgkin's lymphoma. Blood. 1990 Apr 1;75(7):1549–1556. [PubMed] [Google Scholar]
- Kansas G. S., Dailey M. O. Expression of adhesion structures during B cell development in man. J Immunol. 1989 May 1;142(9):3058–3062. [PubMed] [Google Scholar]
- Kansas G. S., Wood G. S., Engleman E. G. Maturational and functional diversity of human B lymphocytes delineated with anti-Leu-8. J Immunol. 1985 May;134(5):3003–3006. [PubMed] [Google Scholar]
- Kataoka T., Oyama A., Suzuki H., Kurita S., Ariyoshi Y., Ota K., Suchi T., Hayashi H., Osada H., Takahashi T. B-cell lymphoma of probable thymic origin: case report. Jpn J Clin Oncol. 1988 Dec;18(4):371–378. [PubMed] [Google Scholar]
- Kimby E., Mellstedt H., Björkholm M., Holm G. Clonal cell surface structures related to differentiation, activation and homing in B-cell chronic lymphocytic leukemia and monoclonal lymphocytosis of undetermined significance. Eur J Haematol. 1989 Nov;43(5):452–459. doi: 10.1111/j.1600-0609.1989.tb00335.x. [DOI] [PubMed] [Google Scholar]
- Lamarre L., Jacobson J. O., Aisenberg A. C., Harris N. L. Primary large cell lymphoma of the mediastinum. A histologic and immunophenotypic study of 29 cases. Am J Surg Pathol. 1989 Sep;13(9):730–739. doi: 10.1097/00000478-198909000-00002. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Loken M. R. Human lymphocyte subpopulations identified by using three-color immunofluorescence and flow cytometry analysis: correlation of Leu-2, Leu-3, Leu-7, Leu-8, and Leu-11 cell surface antigen expression. J Immunol. 1984 Jan;132(1):151–156. [PubMed] [Google Scholar]
- Levitt L. J., Aisenberg A. C., Harris N. L., Linggood R. M., Poppema S. Primary non-Hodgkin's lymphoma of the mediastinum. Cancer. 1982 Dec 1;50(11):2486–2492. doi: 10.1002/1097-0142(19821201)50:11<2486::aid-cncr2820501138>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Lichtenstein A. K., Levine A., Taylor C. R., Boswell W., Rossman S., Feinstein D. I., Lukes R. J. Primary mediastinal lymphoma in adults. Am J Med. 1980 Apr;68(4):509–514. doi: 10.1016/0002-9343(80)90294-6. [DOI] [PubMed] [Google Scholar]
- Loike J. D., Sodeik B., Cao L., Leucona S., Weitz J. I., Detmers P. A., Wright S. D., Silverstein S. C. CD11c/CD18 on neutrophils recognizes a domain at the N terminus of the A alpha chain of fibrinogen. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):1044–1048. doi: 10.1073/pnas.88.3.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlin S. D., Springer T. A. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell. 1987 Dec 4;51(5):813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- Medeiros L. J., Weiss L. M., Picker L. J., Clayberger C., Horning S. J., Krensky A. M., Warnke R. A. Expression of LFA-1 in non-Hodgkin's lymphoma. Cancer. 1989 Jan 15;63(2):255–259. doi: 10.1002/1097-0142(19890115)63:2<255::aid-cncr2820630209>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Menestrina F., Chilosi M., Bonetti F., Lestani M., Scarpa A., Novelli P., Doglioni C., Todeschini G., Ambrosetti A., Fiore-Donati L. Mediastinal large-cell lymphoma of B-type, with sclerosis: histopathological and immunohistochemical study of eight cases. Histopathology. 1986 Jun;10(6):589–600. doi: 10.1111/j.1365-2559.1986.tb02512.x. [DOI] [PubMed] [Google Scholar]
- Michie S. A., Garcia C. F., Strickler J. G., Dailey M. O., Rouse R. V., Warnke R. A. Expression of the Leu-8 antigen by B-cell lymphomas. Am J Clin Pathol. 1987 Oct;88(4):486–490. doi: 10.1093/ajcp/88.4.486. [DOI] [PubMed] [Google Scholar]
- Miedema F., Tromp J. F., van't Veer M. B., Poppema S., Melief C. J. Lymphocyte function-associated antigen 1 (LFA-1) is a marker of mature (immunocompetent) lymphoid cells. A survey of lymphoproliferative diseases in man. Leuk Res. 1985;9(9):1099–1104. doi: 10.1016/0145-2126(85)90098-0. [DOI] [PubMed] [Google Scholar]
- Mielke B., Möller P. Histomorphologic and immunophenotypic spectrum of primary gastro-intestinal B-cell lymphomas. Int J Cancer. 1991 Feb 1;47(3):334–343. doi: 10.1002/ijc.2910470304. [DOI] [PubMed] [Google Scholar]
- Miller J. B., Variakojis D., Bitran J. D., Sweet D. L., Kinzie J. J., Golomb H. M., Ultmann J. E. Diffuse histiocytic lymphoma with sclerosis: a clinicopathologic entity frequently causing superior venacaval obstruction. Cancer. 1981 Feb 15;47(4):748–756. doi: 10.1002/1097-0142(19810215)47:4<748::aid-cncr2820470420>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Momburg F., Herrmann B., Moldenhauer G., Möller P. B-cell lymphomas of high-grade malignancy frequently lack HLA-DR, -DP and -DQ antigens and associated invariant chain. Int J Cancer. 1987 Nov 15;40(5):598–603. doi: 10.1002/ijc.2910400504. [DOI] [PubMed] [Google Scholar]
- Murakawa Y., Strober W., James S. P. Monoclonal antibody against the human peripheral lymph node homing receptor homologue (Leu 8) inhibits B cell differentiation but not B cell proliferation. J Immunol. 1991 Jan 1;146(1):40–46. [PubMed] [Google Scholar]
- Möller P., Eichelmann A., Koretz K., Mechtersheimer G. Adhesion molecules VLA-1 to VLA-6 define discrete stages of peripheral B lymphocyte development and characterize different types of B cell neoplasia. Leukemia. 1992 Apr;6(4):256–264. [PubMed] [Google Scholar]
- Möller P., Eichelmann A., Mechtersheimer G., Koretz K. Expression of beta 1-integrins, H-CAM (CD44) and LECAM-1 in primary gastro-intestinal B-cell lymphomas as compared to the adhesion receptor profile of the gut-associated lymphoid system, tonsil and peripheral lymph node. Int J Cancer. 1991 Dec 2;49(6):846–855. doi: 10.1002/ijc.2910490608. [DOI] [PubMed] [Google Scholar]
- Möller P., Hofmann W. J., Mielke B., Otto H. F. Das primär mediastinale, hellzellige B-Zell-Lymphom ist ein epithelassoziiertes Thymuslymphom. Pathologe. 1989 Jul;10(4):234–239. [PubMed] [Google Scholar]
- Möller P., Lämmler B., Eberlein-Gonska M., Feichter G. E., Hofmann W. J., Schmitteckert H., Otto H. F. Primary mediastinal clear cell lymphoma of B-cell type. Virchows Arch A Pathol Anat Histopathol. 1986;409(1):79–92. doi: 10.1007/BF00705408. [DOI] [PubMed] [Google Scholar]
- Möller P., Matthaei-Maurer D. U., Hofmann W. J., Dörken B., Moldenhauer G. Immunophenotypic similarities of mediastinal clear-cell lymphoma and sinusoidal (monocytoid) B cells. Int J Cancer. 1989 Jan 15;43(1):10–16. doi: 10.1002/ijc.2910430104. [DOI] [PubMed] [Google Scholar]
- Möller P., Moldenhauer G., Momburg F., Lämmler B., Eberlein-Gonska M., Kiesel S., Dörken B. Mediastinal lymphoma of clear cell type is a tumor corresponding to terminal steps of B cell differentiation. Blood. 1987 Apr;69(4):1087–1095. [PubMed] [Google Scholar]
- Nakamine H., Masih A. S., Strobach R. S., Duggan M. J., Bast M. A., Armitage J. O., Weisenburger D. D. Immunoblastic lymphoma with abundant clear cytoplasm. A comparative study of B- and T-cell types. Am J Clin Pathol. 1991 Aug;96(2):177–183. doi: 10.1093/ajcp/96.2.177. [DOI] [PubMed] [Google Scholar]
- Osborn L., Hession C., Tizard R., Vassallo C., Luhowskyj S., Chi-Rosso G., Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989 Dec 22;59(6):1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- Pals S. T., Meijer C. J., Radaszkiewicz T. Expression of the human peripheral lymph node homing receptor (LECAM-1) in nodal and gastrointestinal non-Hodgkin's lymphomas. Leukemia. 1991 Jul;5(7):628–631. [PubMed] [Google Scholar]
- Patarroyo M., Makgoba M. W. Leucocyte adhesion to cells. Molecular basis, physiological relevance, and abnormalities. Scand J Immunol. 1989 Aug;30(2):129–164. doi: 10.1111/j.1365-3083.1989.tb01197.x. [DOI] [PubMed] [Google Scholar]
- Patarroyo M., Prieto J., Ernberg I., Gahmberg C. G. Absence, or low expression, of leukocyte adhesion molecules CD11 and CD18 on Burkitt lymphoma cells. Int J Cancer. 1988 Jun 15;41(6):901–907. doi: 10.1002/ijc.2910410623. [DOI] [PubMed] [Google Scholar]
- Perrone T., Frizzera G., Rosai J. Mediastinal diffuse large-cell lymphoma with sclerosis. A clinicopathologic study of 60 cases. Am J Surg Pathol. 1986 Mar;10(3):176–191. doi: 10.1097/00000478-198603000-00005. [DOI] [PubMed] [Google Scholar]
- Picker L. J., De los Toyos J., Telen M. J., Haynes B. F., Butcher E. C. Monoclonal antibodies against the CD44 [In(Lu)-related p80], and Pgp-1 antigens in man recognize the Hermes class of lymphocyte homing receptors. J Immunol. 1989 Mar 15;142(6):2046–2051. [PubMed] [Google Scholar]
- Picker L. J., Medeiros L. J., Weiss L. M., Warnke R. A., Butcher E. C. Expression of lymphocyte homing receptor antigen in non-Hodgkin's lymphoma. Am J Pathol. 1988 Mar;130(3):496–504. [PMC free article] [PubMed] [Google Scholar]
- Rickinson A. B., Gregory C. D., Young L. S. Viruses and cancer risks: outgrowth of Epstein-Barr virus-positive Burkitt's lymphoma in the immune host. Med Oncol Tumor Pharmacother. 1987;4(3-4):177–186. doi: 10.1007/BF02934513. [DOI] [PubMed] [Google Scholar]
- Rousset F., Billaud M., Blanchard D., Figdor C., Lenoir G. M., Spits H., De Vries J. E. IL-4 induces LFA-1 and LFA-3 expression on Burkitt's lymphoma cell lines. Requirement of additional activation by phorbol myristate acetate for induction of homotypic cell adhesions. J Immunol. 1989 Sep 1;143(5):1490–1498. [PubMed] [Google Scholar]
- Scarpa A., Bonetti F., Menestrina F., Menegazzi M., Chilosi M., Lestani M., Bovolenta C., Zamboni G., Fiore-Donati L. Mediastinal large-cell lymphoma with sclerosis. Genotypic analysis establishes its B nature. Virchows Arch A Pathol Anat Histopathol. 1987;412(1):17–21. doi: 10.1007/BF00750725. [DOI] [PubMed] [Google Scholar]
- Schwarting R., Stein H., Wang C. Y. The monoclonal antibodies alpha S-HCL 1 (alpha Leu-14) and alpha S-HCL 3 (alpha Leu-M5) allow the diagnosis of hairy cell leukemia. Blood. 1985 Apr;65(4):974–983. [PubMed] [Google Scholar]
- Seth R., Salcedo R., Patarroyo M., Makgoba M. W. ICAM-2 peptides mediate lymphocyte adhesion by binding to CD11a/CD18 and CD49d/CD29 integrins. FEBS Lett. 1991 Apr 22;282(1):193–196. doi: 10.1016/0014-5793(91)80475-i. [DOI] [PubMed] [Google Scholar]
- Smith C. W., Marlin S. D., Rothlein R., Toman C., Anderson D. C. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J Clin Invest. 1989 Jun;83(6):2008–2017. doi: 10.1172/JCI114111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spertini O., Kansas G. S., Munro J. M., Griffin J. D., Tedder T. F. Regulation of leukocyte migration by activation of the leukocyte adhesion molecule-1 (LAM-1) selectin. Nature. 1991 Feb 21;349(6311):691–694. doi: 10.1038/349691a0. [DOI] [PubMed] [Google Scholar]
- Stacker S. A., Springer T. A. Leukocyte integrin P150,95 (CD11c/CD18) functions as an adhesion molecule binding to a counter-receptor on stimulated endothelium. J Immunol. 1991 Jan 15;146(2):648–655. [PubMed] [Google Scholar]
- Stamenkovic I., Aruffo A., Amiot M., Seed B. The hematopoietic and epithelial forms of CD44 are distinct polypeptides with different adhesion potentials for hyaluronate-bearing cells. EMBO J. 1991 Feb;10(2):343–348. doi: 10.1002/j.1460-2075.1991.tb07955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauder R., Greil R., Schulz T. F., Thaler J., Gattringer C., Radaskiewicz T., Dierich M. P., Huber H. Expression of leucocyte function-associated antigen-1 and 7F7-antigen, an adhesion molecule related to intercellular adhesion molecule-1 (ICAM-1) in non-Hodgkin lymphomas and leukaemias: possible influence on growth pattern and leukaemic behaviour. Clin Exp Immunol. 1989 Aug;77(2):234–238. [PMC free article] [PubMed] [Google Scholar]
- Staunton D. E., Marlin S. D., Stratowa C., Dustin M. L., Springer T. A. Primary structure of ICAM-1 demonstrates interaction between members of the immunoglobulin and integrin supergene families. Cell. 1988 Mar 25;52(6):925–933. doi: 10.1016/0092-8674(88)90434-5. [DOI] [PubMed] [Google Scholar]
- Strickler J. G., Medeiros L. J., Copenhaver C. M., Weiss L. M., Warnke R. A. Intermediate lymphocytic lymphoma: an immunophenotypic study with comparison to small lymphocytic lymphoma and diffuse small cleaved cell lymphoma. Hum Pathol. 1988 May;19(5):550–554. doi: 10.1016/s0046-8177(88)80203-x. [DOI] [PubMed] [Google Scholar]
- Tedder T. F., Isaacs C. M., Ernst T. J., Demetri G. D., Adler D. A., Disteche C. M. Isolation and chromosomal localization of cDNAs encoding a novel human lymphocyte cell surface molecule, LAM-1. Homology with the mouse lymphocyte homing receptor and other human adhesion proteins. J Exp Med. 1989 Jul 1;170(1):123–133. doi: 10.1084/jem.170.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trump D. L., Mann R. B. Diffuse large cell and undifferentiated lymphomas with prominent mediastinal involvement. Cancer. 1982 Jul 15;50(2):277–282. doi: 10.1002/1097-0142(19820715)50:2<277::aid-cncr2820500218>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Visser L., Shaw A., Slupsky J., Vos H., Poppema S. Monoclonal antibodies reactive with hairy cell leukemia. Blood. 1989 Jul;74(1):320–325. [PubMed] [Google Scholar]
- Waldron J. A., Jr, Dohring E. J., Farber L. R. Primary large cell lymphomas of the mediastinum: an analysis of 20 cases. Semin Diagn Pathol. 1985 Nov;2(4):281–295. [PubMed] [Google Scholar]
- Walter J., Möller P., Moldenhauer G., Schirrmacher V., Pawlita M., Wolf J. Local growth of a Burkitt's lymphoma versus disseminated invasive growth of the autologous EBV-immortalized lymphoblastoid cells and their somatic cell hybrids in SCID mice. Int J Cancer. 1992 Jan 21;50(2):265–273. doi: 10.1002/ijc.2910500217. [DOI] [PubMed] [Google Scholar]
- Yousem S. A., Weiss L. M., Warnke R. A. Primary mediastinal non-Hodgkin's lymphomas: a morphologic and immunologic study of 19 cases. Am J Clin Pathol. 1985 Jun;83(6):676–680. doi: 10.1093/ajcp/83.6.676. [DOI] [PubMed] [Google Scholar]
- al-Sharabati M., Chittal S., Duga-Neulat I., Laurent G., Mazerolles C., al-Saati T., Brousset P., Delsol G. Primary anterior mediastinal B-cell lymphoma. A clinicopathologic and immunohistochemical study of 16 cases. Cancer. 1991 May 15;67(10):2579–2587. doi: 10.1002/1097-0142(19910515)67:10<2579::aid-cncr2820671030>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]