Abstract
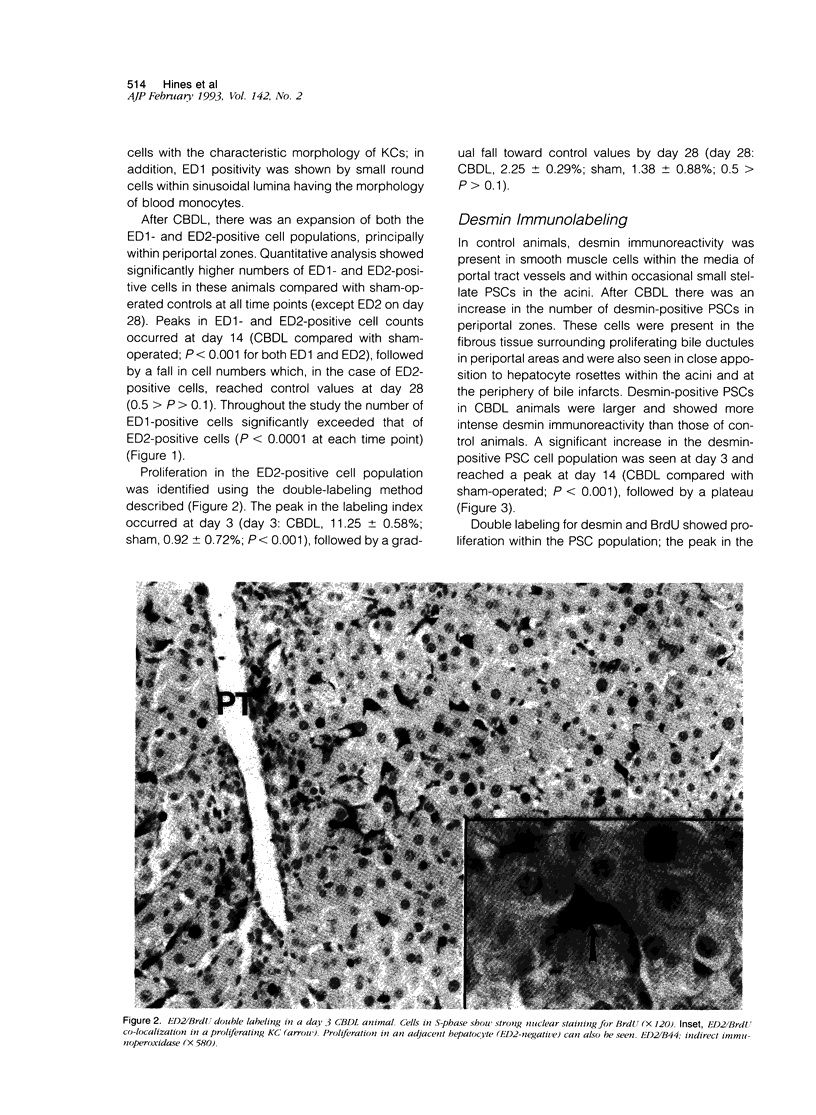
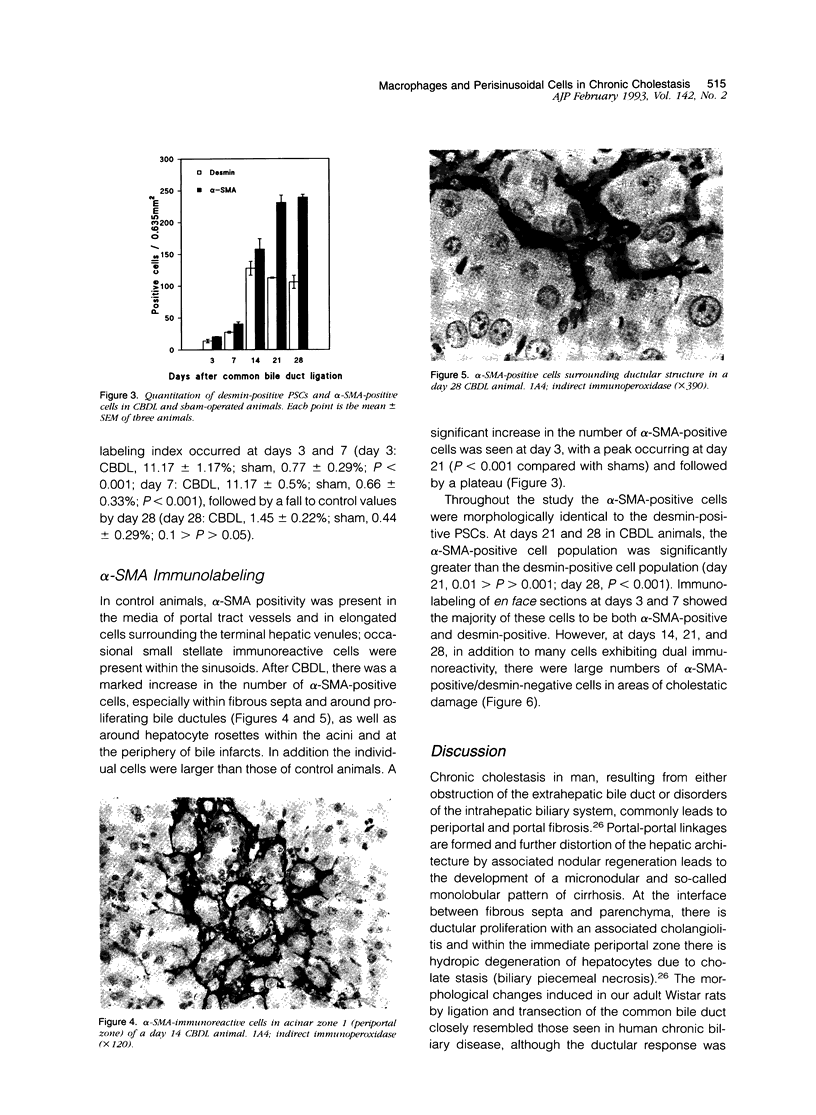
We investigated the response of macrophages and perisinusoidal (Ito) cells (PSCs) during the development of secondary biliary cirrhosis after ligation and division of the common bile duct. Liver tissue was obtained from three groups of male Wistar rats: 1) untreated controls (n = 3); 2) common bile duct-ligated (CBDL) animals (n = 15); and 3) sham-operated controls (n = 15). Material from animal groups 2 and 3 was obtained on days 3, 7, 14, 21, and 28 after operation; in all animals 5-bromo-2-deoxyuridine was administered intraperitoneally before death. Monocytes and macrophages were detected using the monoclonal antibody ED1 and tissue macrophages using the antibody ED2. Cell proliferation within the macrophage population was demonstrated by double labeling for ED2 and incorporated 5-bromo-2-deoxyuridine. PSCs were demonstrated in tissue sections by immunolocalization of desmin; proliferating PSCs were identified by double labeling for desmin and incorporated 5-bromo-2-deoxyuridine. Evidence of phenotypic modulation of PSCs was sought using anti-alpha-smooth muscle actin (alpha-SMA) antibody. Increased numbers of ED1- and ED2-positive cells were seen in CBDL animals at all time points. Local proliferation of macrophages could be identified and reached a peak at day 3, thereafter falling toward control values. Compared with those of controls, livers of CBDL animals showed increased numbers of desmin-positive PSCs in periportal zones from day 3 on, reaching a peak at day 14 (127.8 +/- 10.99 cells/0.635 mm2) and followed by a plateau. PSC proliferation peaked at days 3 and 7 (labeling indices 11.2% and 11.2%, respectively) and thereafter fell toward control values; no expansion of the PSC population was seen in sham-operated rats. Increased alpha-SMA-positive cells were also noted from day 3, with a peak at day 21 (231.1 +/- 11.52 cells/0.635 mm2) and followed by a plateau. En face labeling experiments in days 14, 21, and 28 CBDL animals showed cells co-expressing alpha-SMA and desmin and cells expressing alpha-SMA alone. These results indicate that in response to chronic cholestatic liver injury, PSCs proliferate and undergo phenotypic modulation toward "myofibroblast-like" cells. The kinetics of the response are similar to those of the ED2-positive cell population in keeping with a hypothesis that PSC proliferation and activation may be mediated by factors released by macrophages in response to various forms of liver injury. We conclude that the responses of macrophages and PSCs to cholestatic injury are similar to those after toxin-induced hepatocyte necrosis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Aziz G., Lebeau G., Rescan P. Y., Clément B., Rissel M., Deugnier Y., Campion J. P., Guillouzo A. Reversibility of hepatic fibrosis in experimentally induced cholestasis in rat. Am J Pathol. 1990 Dec;137(6):1333–1342. [PMC free article] [PubMed] [Google Scholar]
- Abdel-Aziz G., Rescan P. Y., Clement B., Lebeau G., Rissel M., Grimaud J. A., Campion J. P., Guillouzo A. Cellular sources of matrix proteins in experimentally induced cholestatic rat liver. J Pathol. 1991 Jun;164(2):167–174. doi: 10.1002/path.1711640211. [DOI] [PubMed] [Google Scholar]
- Arthur M. J. Matrix degradation in the liver. Semin Liver Dis. 1990 Feb;10(1):47–55. doi: 10.1055/s-2008-1040456. [DOI] [PubMed] [Google Scholar]
- Ballardini G., Fallani M., Biagini G., Bianchi F. B., Pisi E. Desmin and actin in the identification of Ito cells and in monitoring their evolution to myofibroblasts in experimental liver fibrosis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1988;56(1):45–49. doi: 10.1007/BF02890000. [DOI] [PubMed] [Google Scholar]
- Bedossa P., Lemaigre G., Paraf F., Martin E. Deposition and remodelling of elastic fibres in chronic hepatitis. Virchows Arch A Pathol Anat Histopathol. 1990;417(2):159–162. doi: 10.1007/BF02190534. [DOI] [PubMed] [Google Scholar]
- Bissell D. M., Friedman S. L., Maher J. J., Roll F. J. Connective tissue biology and hepatic fibrosis: report of a conference. Hepatology. 1990 Mar;11(3):488–498. doi: 10.1002/hep.1840110322. [DOI] [PubMed] [Google Scholar]
- Burt A. D., Robertson J. L., Heir J., MacSween R. N. Desmin-containing stellate cells in rat liver; distribution in normal animals and response to experimental acute liver injury. J Pathol. 1986 Sep;150(1):29–35. doi: 10.1002/path.1711500106. [DOI] [PubMed] [Google Scholar]
- Chojkier M., Lyche K. D., Filip M. Increased production of collagen in vivo by hepatocytes and nonparenchymal cells in rats with carbon tetrachloride-induced hepatic fibrosis. Hepatology. 1988 Jul-Aug;8(4):808–814. doi: 10.1002/hep.1840080419. [DOI] [PubMed] [Google Scholar]
- Clément B., Rescan P. Y., Baffet G., Loréal O., Lehry D., Campion J. P., Guillouzo A. Hepatocytes may produce laminin in fibrotic liver and in primary culture. Hepatology. 1988 Jul-Aug;8(4):794–803. doi: 10.1002/hep.1840080417. [DOI] [PubMed] [Google Scholar]
- Evarts R. P., Nakatsukasa H., Marsden E. R., Hsia C. C., Dunsford H. A., Thorgeirsson S. S. Cellular and molecular changes in the early stages of chemical hepatocarcinogenesis in the rat. Cancer Res. 1990 Jun 1;50(11):3439–3444. [PubMed] [Google Scholar]
- Friedman S. L. Cellular sources of collagen and regulation of collagen production in liver. Semin Liver Dis. 1990 Feb;10(1):20–29. doi: 10.1055/s-2008-1040454. [DOI] [PubMed] [Google Scholar]
- Geerts A., Lazou J. M., De Bleser P., Wisse E. Tissue distribution, quantitation and proliferation kinetics of fat-storing cells in carbon tetrachloride-injured rat liver. Hepatology. 1991 Jun;13(6):1193–1202. [PubMed] [Google Scholar]
- Gressner A. M., Bachem M. G. Cellular sources of noncollagenous matrix proteins: role of fat-storing cells in fibrogenesis. Semin Liver Dis. 1990 Feb;10(1):30–46. doi: 10.1055/s-2008-1040455. [DOI] [PubMed] [Google Scholar]
- Johnson S. J., Hines J. E., Burt A. D. Immunolocalization of proliferating perisinusoidal cells in rat liver. Histochem J. 1992 Feb;24(2):67–72. doi: 10.1007/BF01082441. [DOI] [PubMed] [Google Scholar]
- Johnson S. J., Hines J. E., Burt A. D. Macrophage and perisinusoidal cell kinetics in acute liver injury. J Pathol. 1992 Apr;166(4):351–358. doi: 10.1002/path.1711660406. [DOI] [PubMed] [Google Scholar]
- Jonker A. M., Dijkhuis F. W., Kroese F. G., Hardonk M. J., Grond J. Immunopathology of acute galactosamine hepatitis in rats. Hepatology. 1990 Apr;11(4):622–627. doi: 10.1002/hep.1840110415. [DOI] [PubMed] [Google Scholar]
- Jézéquel A. M., Mancini R., Rinaldesi M. L., Ballardini G., Fallani M., Bianchi F., Orlandi F. Dimethylnitrosamine-induced cirrhosis. Evidence for an immunological mechanism. J Hepatol. 1989 Jan;8(1):42–52. doi: 10.1016/0168-8278(89)90160-8. [DOI] [PubMed] [Google Scholar]
- Kountouras J., Billing B. H., Scheuer P. J. Prolonged bile duct obstruction: a new experimental model for cirrhosis in the rat. Br J Exp Pathol. 1984 Jun;65(3):305–311. [PMC free article] [PubMed] [Google Scholar]
- Matsuoka M., Pham N. T., Tsukamoto H. Differential effects of interleukin-1 alpha, tumor necrosis factor alpha, and transforming growth factor beta 1 on cell proliferation and collagen formation by cultured fat-storing cells. Liver. 1989 Apr;9(2):71–78. doi: 10.1111/j.1600-0676.1989.tb00382.x. [DOI] [PubMed] [Google Scholar]
- Matsuoka M., Tsukamoto H. Stimulation of hepatic lipocyte collagen production by Kupffer cell-derived transforming growth factor beta: implication for a pathogenetic role in alcoholic liver fibrogenesis. Hepatology. 1990 Apr;11(4):599–605. doi: 10.1002/hep.1840110412. [DOI] [PubMed] [Google Scholar]
- McGee J. O., Patrick R. S. The role of perisinusoidal cells in hepatic fibrogenesis. An electron microscopic study of acute carbon tetrachloride liver injury. Lab Invest. 1972 Apr;26(4):429–440. [PubMed] [Google Scholar]
- Milani S., Herbst H., Schuppan D., Kim K. Y., Riecken E. O., Stein H. Procollagen expression by nonparenchymal rat liver cells in experimental biliary fibrosis. Gastroenterology. 1990 Jan;98(1):175–184. doi: 10.1016/0016-5085(90)91307-r. [DOI] [PubMed] [Google Scholar]
- Pinzani M., Gesualdo L., Sabbah G. M., Abboud H. E. Effects of platelet-derived growth factor and other polypeptide mitogens on DNA synthesis and growth of cultured rat liver fat-storing cells. J Clin Invest. 1989 Dec;84(6):1786–1793. doi: 10.1172/JCI114363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadori G., Veit T., Schwögler S., Dienes H. P., Knittel T., Rieder H., Meyer zum Büschenfelde K. H. Expression of the gene of the alpha-smooth muscle-actin isoform in rat liver and in rat fat-storing (ITO) cells. Virchows Arch B Cell Pathol Incl Mol Pathol. 1990;59(6):349–357. doi: 10.1007/BF02899424. [DOI] [PubMed] [Google Scholar]
- Schuppan D. Structure of the extracellular matrix in normal and fibrotic liver: collagens and glycoproteins. Semin Liver Dis. 1990 Feb;10(1):1–10. doi: 10.1055/s-2008-1040452. [DOI] [PubMed] [Google Scholar]
- Shiratori Y., Geerts A., Ichida T., Kawase T., Wisse E. Kupffer cells from CCl4-induced fibrotic livers stimulate proliferation of fat-storing cells. J Hepatol. 1986;3(3):294–303. doi: 10.1016/s0168-8278(86)80481-0. [DOI] [PubMed] [Google Scholar]
- Skalli O., Ropraz P., Trzeciak A., Benzonana G., Gillessen D., Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986 Dec;103(6 Pt 2):2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Nouchi T., Yamane M., Irie T., Miyakawa H., Sato C., Marumo F. Phenotypic modulation in lipocytes in experimental liver fibrosis. J Pathol. 1991 Jul;164(3):273–278. doi: 10.1002/path.1711640314. [DOI] [PubMed] [Google Scholar]
- Van Eyken P., Sciot R., Desmet V. J. Expression of the novel extracellular matrix component tenascin in normal and diseased human liver. An immunohistochemical study. J Hepatol. 1990 Jul;11(1):43–52. doi: 10.1016/0168-8278(90)90270-2. [DOI] [PubMed] [Google Scholar]
- Weiner F. R., Giambrone M. A., Czaja M. J., Shah A., Annoni G., Takahashi S., Eghbali M., Zern M. A. Ito-cell gene expression and collagen regulation. Hepatology. 1990 Jan;11(1):111–117. doi: 10.1002/hep.1840110119. [DOI] [PubMed] [Google Scholar]
- Yokoi Y., Namihisa T., Kuroda H., Komatsu I., Miyazaki A., Watanabe S., Usui K. Immunocytochemical detection of desmin in fat-storing cells (Ito cells). Hepatology. 1984 Jul-Aug;4(4):709–714. doi: 10.1002/hep.1840040425. [DOI] [PubMed] [Google Scholar]