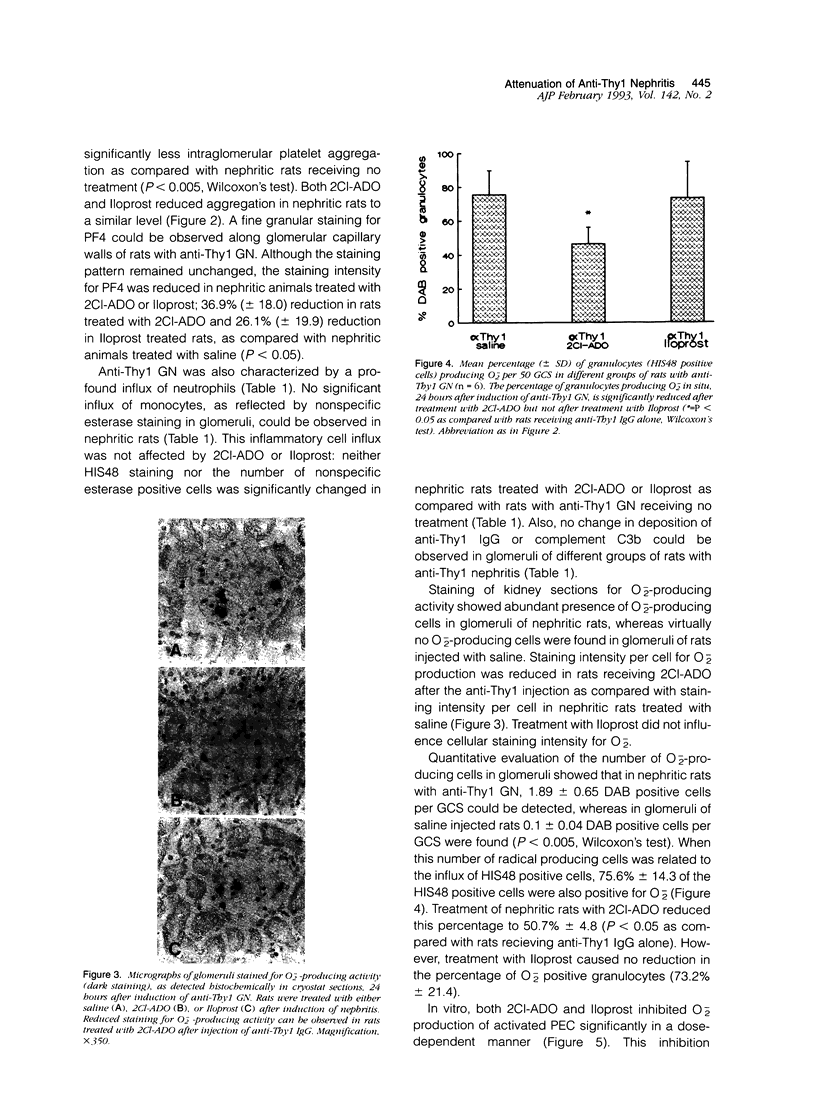
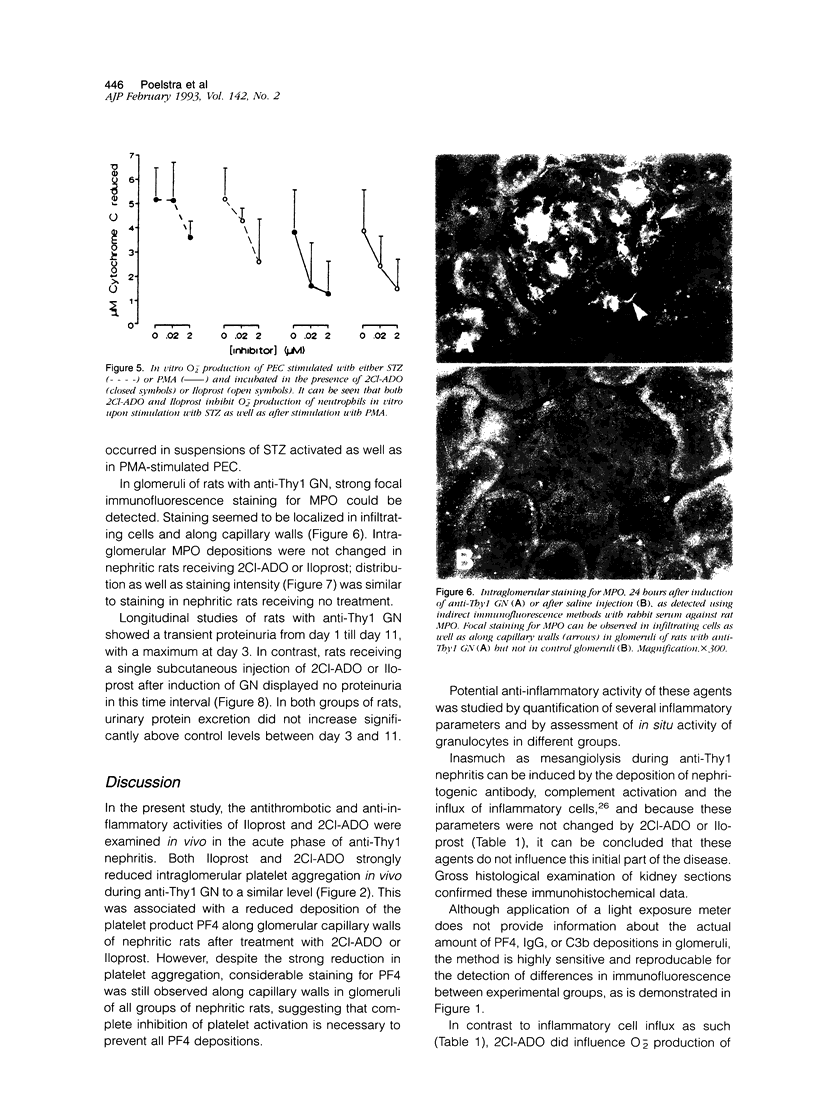
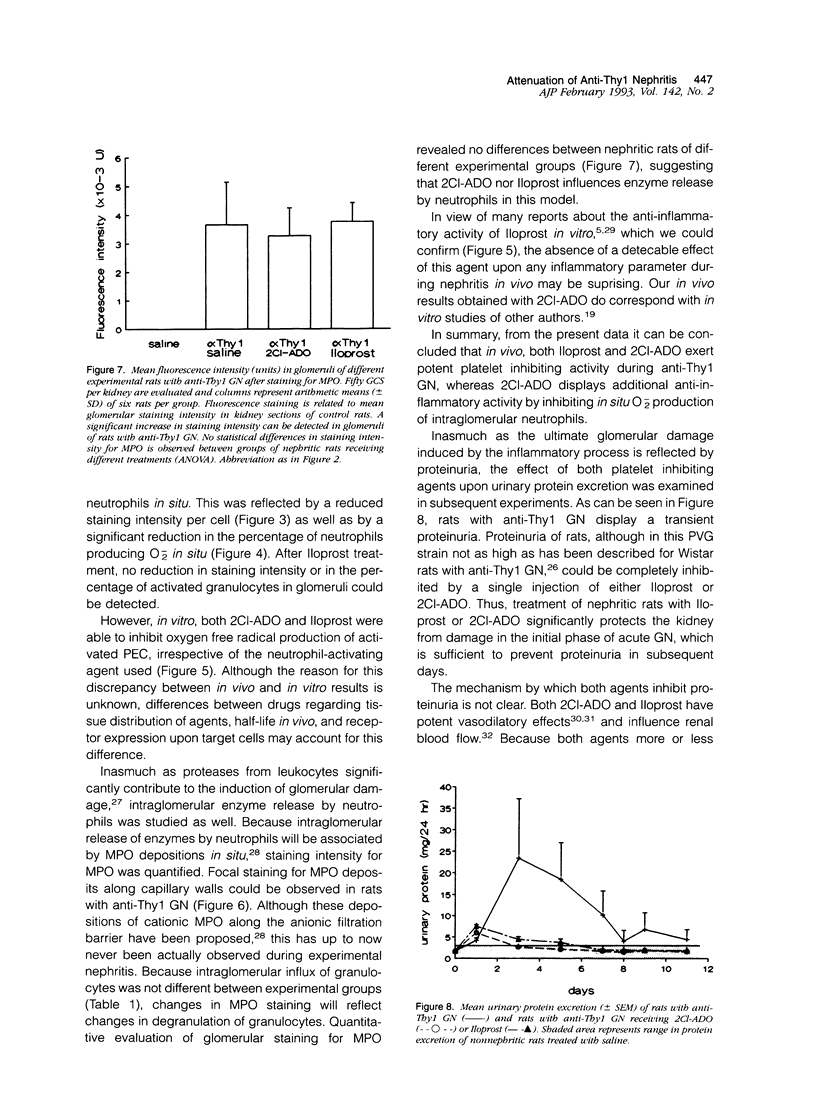
Abstract
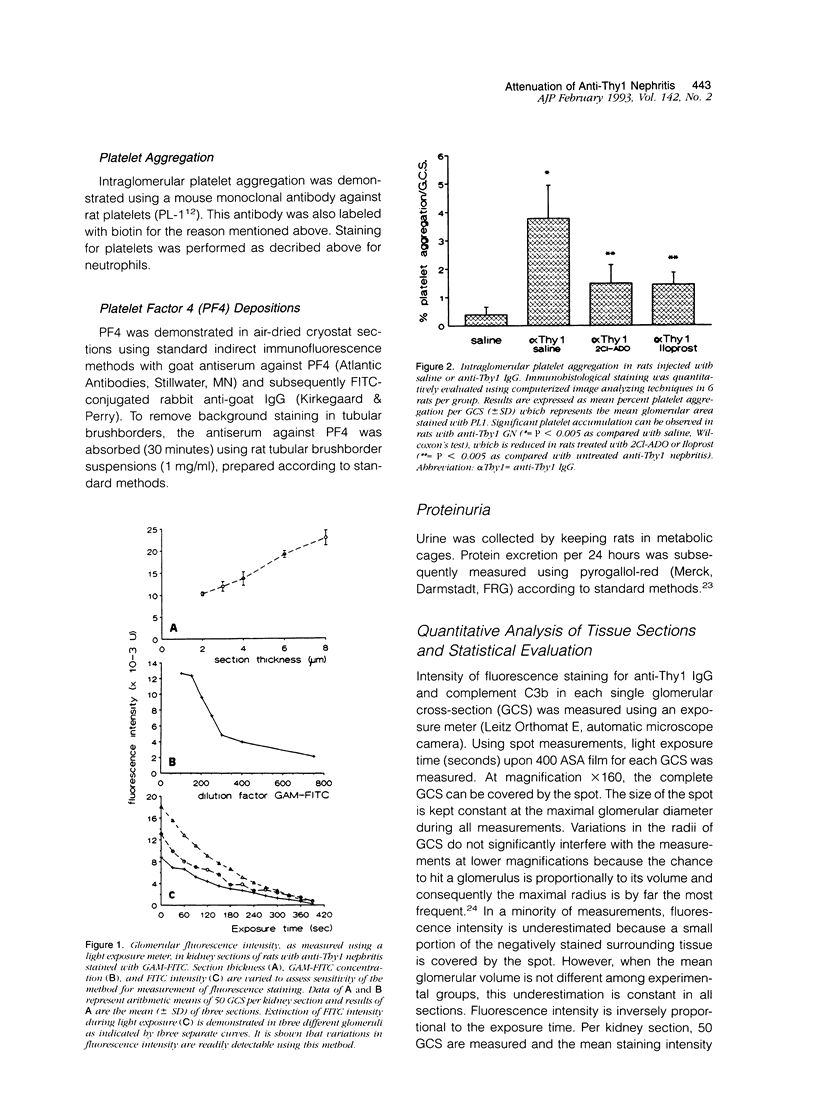
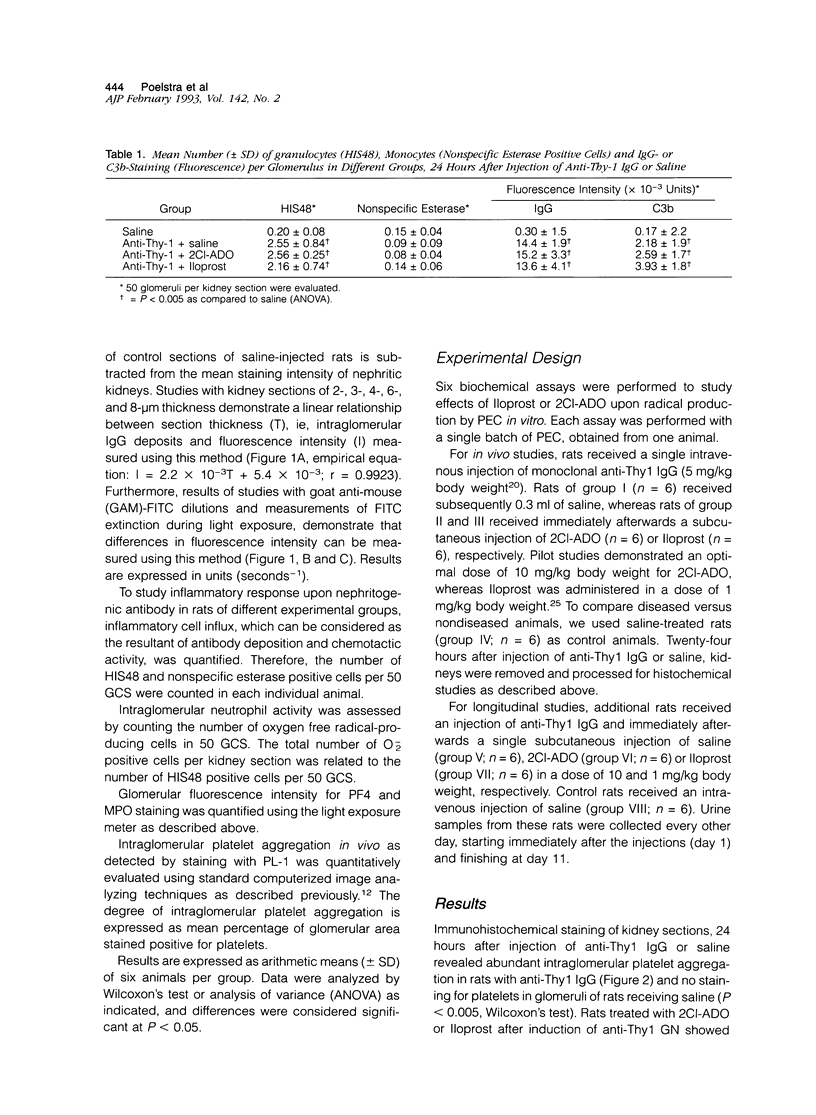
Although both ecto-ADPase and prostacyclin (PGI2) inhibit platelets and neutrophils, their action in acute glomerulonephritis is unknown. We tested the PGI2 analog Iloprost and 2chloroadenosine (2Cl-ADO), an analog of adenosine, the end product of nucleotidase activities, during anti-Thy1 nephritis. Rats received anti-Thy1 immunoglobulin G (5 mg/kg body weight, intravenously) and subsequently one subcutaneous injection of either 2Cl-ADO (10 mg/kg body weight; (n = 6) or Iloprost (1 mg/kg body weight; n = 6). Control rats received anti-Thy1 immunoglobulin G with saline (n = 6) or saline alone (n = 6). After 24 hours, kidneys were processed for light-microscopical evaluation. Proteinuria was studied in additional rats. Results showed that both drugs inhibited intraglomerular platelet activation (P < 0.005). 2Cl-ADO also reduced intraglomerular O2- production of neutrophils (P < 0.05), in contrast to Iloprost. Intraglomerular immunoglobulin G deposition, complement activation, neutrophil influx, and myeloperoxidase release were not affected by 2Cl-ADO or Iloprost. However, proteinuria was completely prevented by both drugs. It is concluded that PGI2 and nucleotidases are potentially able to attenuate this form of nephritis by inhibiting platelet activity, whereas nucleotidases also inhibit neutrophil activity in vivo.
Full text
PDF




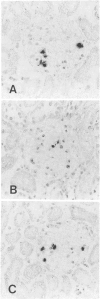
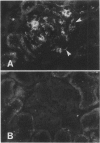
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagchus W. M., Hoedemaeker P. J., Rozing J., Bakker W. W. Acute glomerulonephritis after intravenous injection of monoclonal anti-thymocyte antibodies in the rat. Immunol Lett. 1986 Mar;12(2-3):109–113. doi: 10.1016/0165-2478(86)90091-x. [DOI] [PubMed] [Google Scholar]
- Bakker W. W., Kalicharan D., Donga J., Hulstaert C. E., Hardonk M. J. Decreased ATPase activity in adriamycin nephrosis is independent of proteinuria. Kidney Int. 1987 Mar;31(3):704–709. doi: 10.1038/ki.1987.55. [DOI] [PubMed] [Google Scholar]
- Bakker W. W., Willink E. J., Donga J., Hulstaert C. E., Hardonk M. J. Antithrombotic activity of glomerular adenosine diphosphatase in the glomerular basement membrane of the rat kidney. J Lab Clin Med. 1987 Feb;109(2):171–177. [PubMed] [Google Scholar]
- Barnes J. L., Levine S. P., Venkatachalam M. A. Binding of platelet factor four (PF 4) to glomerular polyanion. Kidney Int. 1984 May;25(5):759–765. doi: 10.1038/ki.1984.87. [DOI] [PubMed] [Google Scholar]
- Boxer L. A., Allen J. M., Schmidt M., Yoder M., Baehner R. L. Inhibition of polymorphonuclear leukocyte adherence by prostacyclin. J Lab Clin Med. 1980 May;95(5):672–678. [PubMed] [Google Scholar]
- Camussi G., Tetta C., Coda R., Segoloni G. P., Vercellone A. Platelet-activating factor-induced loss of glomerular anionic charges. Kidney Int. 1984 Jan;25(1):73–81. doi: 10.1038/ki.1984.10. [DOI] [PubMed] [Google Scholar]
- Clark W. F., Parbtani A., McDonald J. W., Taylor N., Reid B. D., Kreeft J. The effects of a thromboxane synthase inhibitor, a prostacyclin analog and PGE1 on the nephritis of the NZB/W F1 mouse. Clin Nephrol. 1987 Dec;28(6):288–294. [PubMed] [Google Scholar]
- Cronstein B. N., Levin R. I., Belanoff J., Weissmann G., Hirschhorn R. Adenosine: an endogenous inhibitor of neutrophil-mediated injury to endothelial cells. J Clin Invest. 1986 Sep;78(3):760–770. doi: 10.1172/JCI112638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber J. G., Payne N. A., Murphy R. C., Nies A. S. Prostacyclin produced by the pregnant uterus in the dog may act as a circulating vasodepressor substance. J Clin Invest. 1981 Mar;67(3):632–636. doi: 10.1172/JCI110077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow J. G., Schade R., Pitlick F. A. Evidence that ADP hydrolysis by human cells is related to thrombogenic potential1. Thromb Res. 1978 Aug;13(2):255–266. doi: 10.1016/0049-3848(78)90013-0. [DOI] [PubMed] [Google Scholar]
- Gordon E. L., Pearson J. D., Slakey L. L. The hydrolysis of extracellular adenine nucleotides by cultured endothelial cells from pig aorta. Feed-forward inhibition of adenosine production at the cell surface. J Biol Chem. 1986 Nov 25;261(33):15496–15507. [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardonk M. J., Kalicharan D., Hulstaert C. E. Cytochemical demonstration of ATPase activity in the rat kidney basement membrane using the cerium-based method. Acta Histochem Suppl. 1985;31:253–261. [PubMed] [Google Scholar]
- Haurand M., Flohé L. Leukotriene formation by human polymorphonuclear leukocytes from endogenous arachidonate. Physiological triggers and modulation by prostanoids. Biochem Pharmacol. 1989 Jul 1;38(13):2129–2137. doi: 10.1016/0006-2952(89)90067-1. [DOI] [PubMed] [Google Scholar]
- Hopwood A. M., Lincoln J., Kirkpatrick K. A., Burnstock G. Adenosine 5'-triphosphate, adenosine and endothelium-derived relaxing factor in hypoxic vasodilatation of the heart. Eur J Pharmacol. 1989 Jun 20;165(2-3):323–326. doi: 10.1016/0014-2999(89)90730-9. [DOI] [PubMed] [Google Scholar]
- Johnson R. J., Alpers C. E., Pritzl P., Schulze M., Baker P., Pruchno C., Couser W. G. Platelets mediate neutrophil-dependent immune complex nephritis in the rat. J Clin Invest. 1988 Oct;82(4):1225–1235. doi: 10.1172/JCI113720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. J., Alpers C. E., Pruchno C., Schulze M., Baker P. J., Pritzl P., Couser W. G. Mechanisms and kinetics for platelet and neutrophil localization in immune complex nephritis. Kidney Int. 1989 Nov;36(5):780–789. doi: 10.1038/ki.1989.263. [DOI] [PubMed] [Google Scholar]
- Johnson R. J., Couser W. G., Chi E. Y., Adler S., Klebanoff S. J. New mechanism for glomerular injury. Myeloperoxidase-hydrogen peroxide-halide system. J Clin Invest. 1987 May;79(5):1379–1387. doi: 10.1172/JCI112965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. J., Garcia R. L., Pritzl P., Alpers C. E. Platelets mediate glomerular cell proliferation in immune complex nephritis induced by anti-mesangial cell antibodies in the rat. Am J Pathol. 1990 Feb;136(2):369–374. [PMC free article] [PubMed] [Google Scholar]
- Johnson R. J., Raines E. W., Floege J., Yoshimura A., Pritzl P., Alpers C., Ross R. Inhibition of mesangial cell proliferation and matrix expansion in glomerulonephritis in the rat by antibody to platelet-derived growth factor. J Exp Med. 1992 May 1;175(5):1413–1416. doi: 10.1084/jem.175.5.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhns D. B., Wright D. G., Nath J., Kaplan S. S., Basford R. E. ATP induces transient elevations of [Ca2+]i in human neutrophils and primes these cells for enhanced O2- generation. Lab Invest. 1988 Apr;58(4):448–453. [PubMed] [Google Scholar]
- Lieberman G. E., Lewis G. P., Peters T. J. A membrane-bound enzyme in rabbit aorta capable of inhibiting adenosine-diphosphate-induced platelet aggregation. Lancet. 1977 Aug 13;2(8033):330–332. doi: 10.1016/s0140-6736(77)91488-x. [DOI] [PubMed] [Google Scholar]
- Margaretten W., McKay D. G. The requirement for platelets in the active Arthus reaction. Am J Pathol. 1971 Aug;64(2):257–270. [PMC free article] [PubMed] [Google Scholar]
- Merrill D. P. Purification of human myeloperoxidase by Concanavalin A-Sepharose affinity chromatography. Prep Biochem. 1980;10(2):133–50. doi: 10.1080/00327488008061729. [DOI] [PubMed] [Google Scholar]
- Poelstra K., Baller J. F., Hardonk M. J., Bakker W. W. Demonstration of antithrombotic activity of glomerular adenosine diphosphatase. Blood. 1991 Jul 1;78(1):141–148. [PubMed] [Google Scholar]
- Poelstra K., Baller J. F., Hardonk M. J., Bakker W. W. Intraglomerular thrombotic tendency and glomerular ADPase. Unilateral impairment of ADPase elicits a proaggregatory microenvironment in experimental glomerulonephritis. Lab Invest. 1991 Apr;64(4):520–526. [PubMed] [Google Scholar]
- Poelstra K., Hardonk M. J., Koudstaal J., Bakker W. W. Intraglomerular platelet aggregation and experimental glomerulonephritis. Kidney Int. 1990 Jun;37(6):1500–1508. doi: 10.1038/ki.1990.141. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. The anti-aggregating properties of vascular endothelium: interactions between prostacyclin and nitric oxide. Br J Pharmacol. 1987 Nov;92(3):639–646. doi: 10.1111/j.1476-5381.1987.tb11367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrijver G., Schalkwijk J., Robben J. C., Assmann K. J., Koene R. A. Antiglomerular basement membrane nephritis in beige mice. Deficiency of leukocytic neutral proteinases prevents the induction of albuminuria in the heterologous phase. J Exp Med. 1989 Apr 1;169(4):1435–1448. doi: 10.1084/jem.169.4.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson P. J., Mickelson J., Fantone J. C., Gallagher K. P., Lucchesi B. R. Iloprost inhibits neutrophil function in vitro and in vivo and limits experimental infarct size in canine heart. Circ Res. 1987 May;60(5):666–673. doi: 10.1161/01.res.60.5.666. [DOI] [PubMed] [Google Scholar]
- Tetta C., Coda R., Camussi G. Human platelet cationic proteins bind to rat glomeruli, induce loss of anionic charges and increase glomerular permeability. Agents Actions. 1985 Mar;16(1-2):24–26. doi: 10.1007/BF01999634. [DOI] [PubMed] [Google Scholar]
- Varani J., Phan S. H., Gibbs D. F., Ryan U. S., Ward P. A. H2O2-mediated cytotoxicity of rat pulmonary endothelial cells. Changes in adenosine triphosphate and purine products and effects of protective interventions. Lab Invest. 1990 Nov;63(5):683–689. [PubMed] [Google Scholar]
- Ward P. A., Cunningham T. W., McCulloch K. K., Johnson K. J. Regulatory effects of adenosine and adenine nucleotides on oxygen radical responses of neutrophils. Lab Invest. 1988 Apr;58(4):438–447. [PubMed] [Google Scholar]
- Ward P. A., Cunningham T. W., McCulloch K. K., Phan S. H., Powell J., Johnson K. J. Platelet enhancement of O2-. responses in stimulated human neutrophils. Identification of platelet factor as adenine nucleotide. Lab Invest. 1988 Jan;58(1):37–47. [PubMed] [Google Scholar]
- Watanabe N., Kamei S., Ohkubo A., Yamanaka M., Ohsawa S., Makino K., Tokuda K. Urinary protein as measured with a pyrogallol red-molybdate complex, manually and in a Hitachi 726 automated analyzer. Clin Chem. 1986 Aug;32(8):1551–1554. [PubMed] [Google Scholar]
- van Bamme B., Koudstaal J. Measuring glomerular diameters in tissue sections. Virchows Arch A Pathol Anat Histol. 1976 Mar 5;369(4):283–291. doi: 10.1007/BF00432450. [DOI] [PubMed] [Google Scholar]
- van Goor H., Fidler V., Weening J. J., Grond J. Determinants of focal and segmental glomerulosclerosis in the rat after renal ablation. Evidence for involvement of macrophages and lipids. Lab Invest. 1991 Jun;64(6):754–765. [PubMed] [Google Scholar]
- van Waeg G., Van den Berghe G. Purine catabolism in polymorphonuclear neutrophils. Phorbol myristate acetate-induced accumulation of adenosine owing to inactivation of extracellularly released adenosine deaminase. J Clin Invest. 1991 Jan;87(1):305–312. doi: 10.1172/JCI114987. [DOI] [PMC free article] [PubMed] [Google Scholar]