Abstract
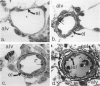
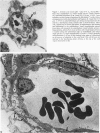
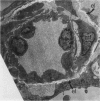
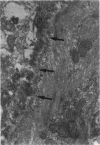
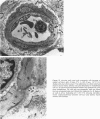
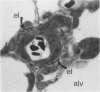
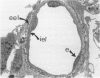
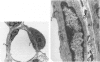
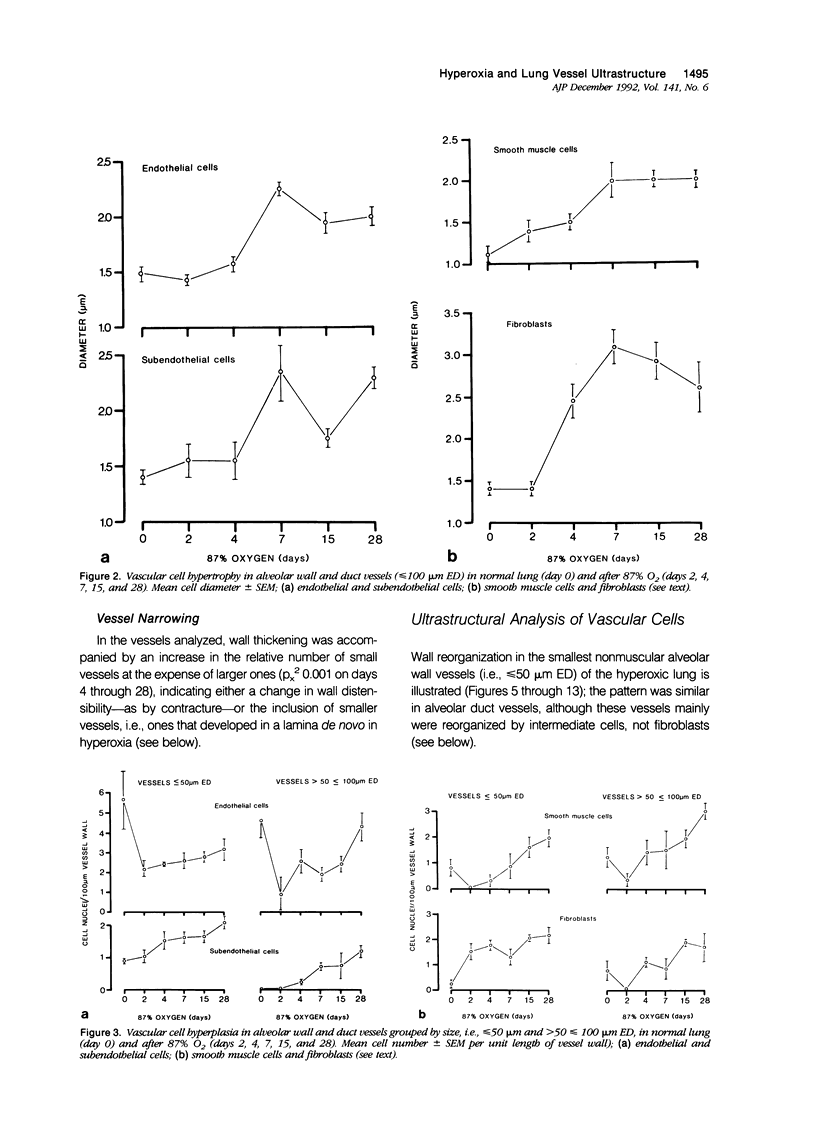
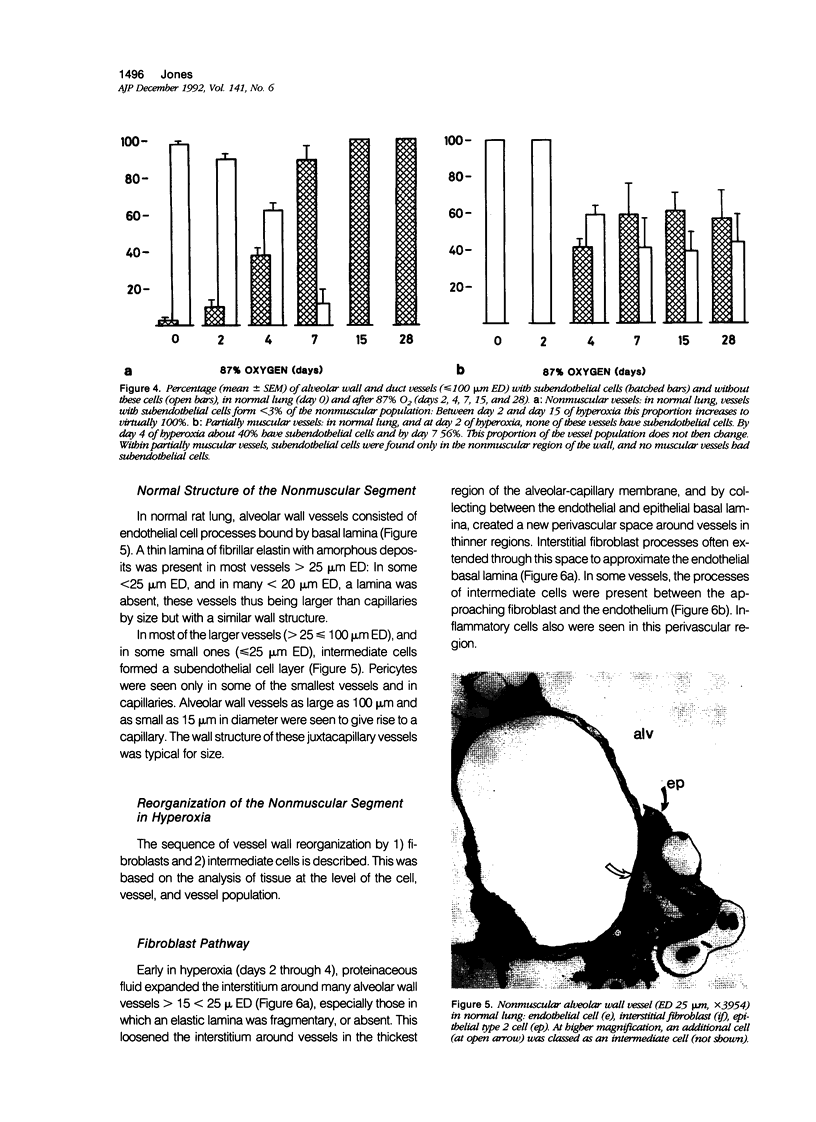
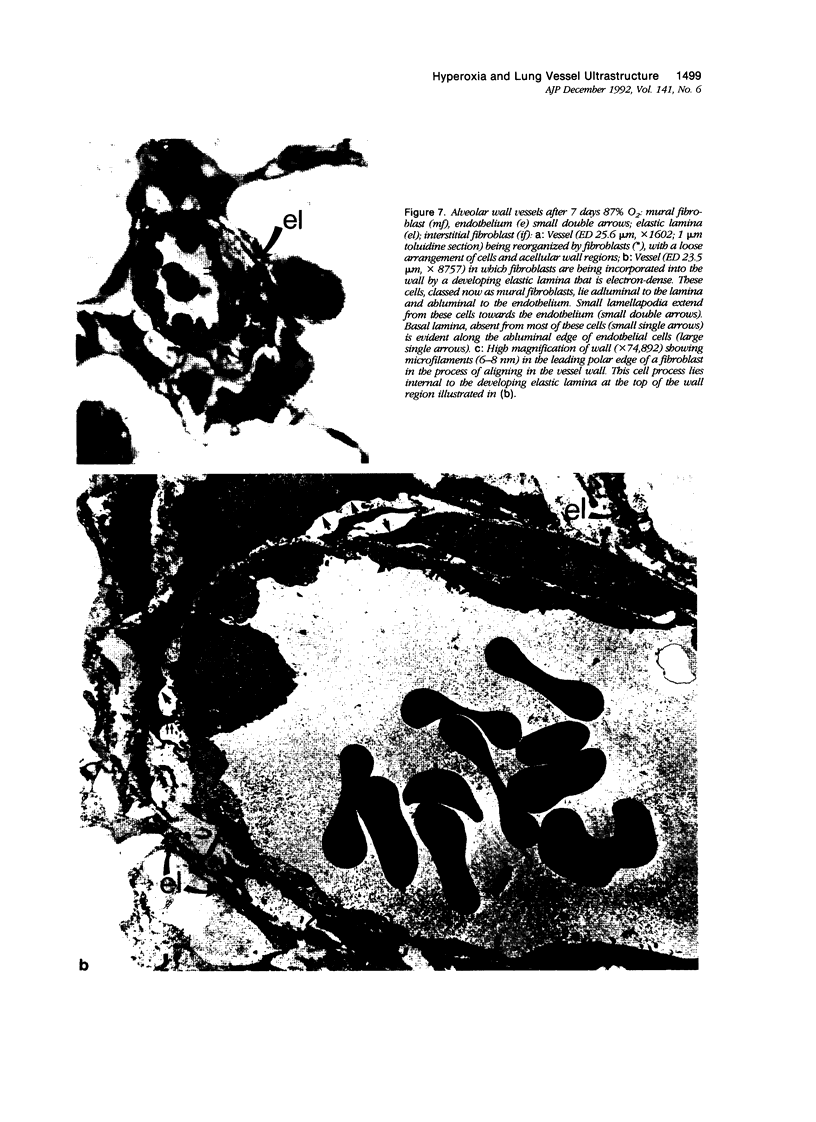
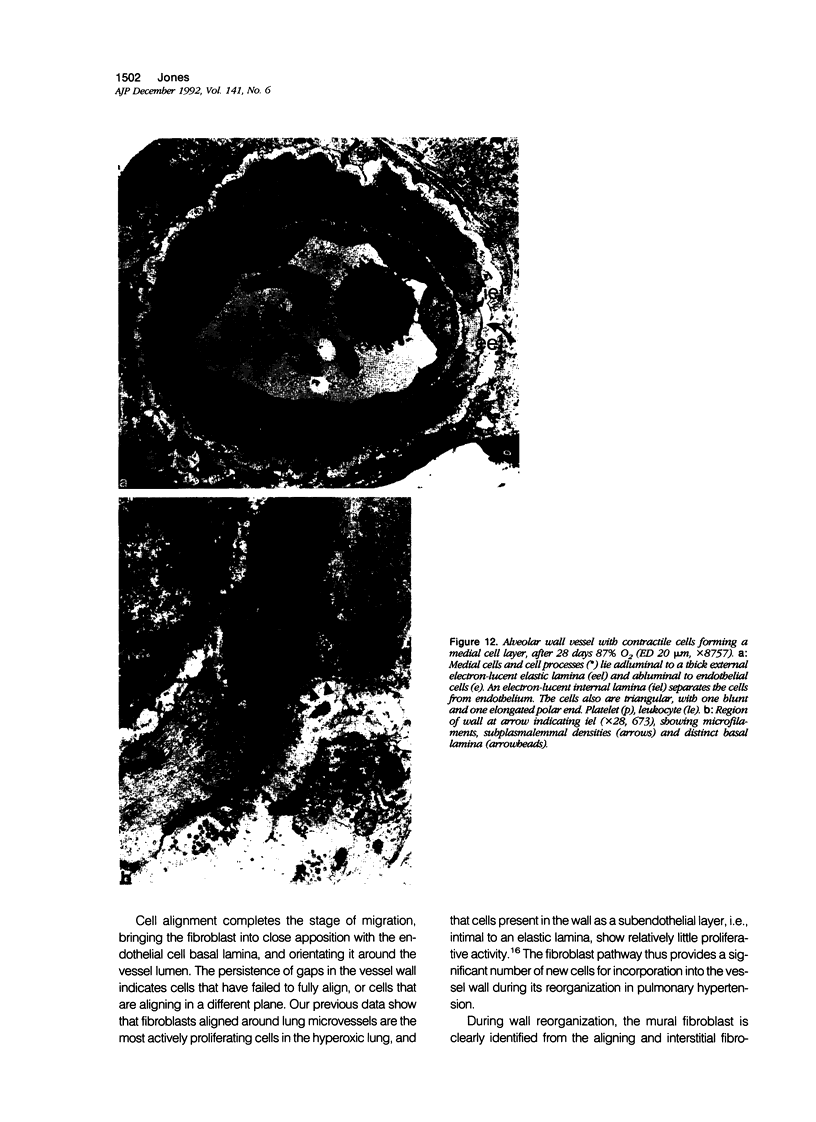
The current study traces the development of contractile cells in the nonmuscular segments of rat lung microvessels in hyperoxic pulmonary hypertension. New intimal cells first develop into a well-defined layer beneath the endothelium and internal to an elastic lamina. Ultrastructurally, these cells are found to be 1) fibroblasts recruited to the vessel wall from the interstitium and 2) intermediate cells, a population of preexisting vascular cells (structurally between a smooth muscle cell and a pericyte). Early in hyperoxia (days 3 through 7), interstitial fibroblasts migrate and align around the smallest vessels in which an elastic lamina is either absent or fragmentary. These cells then are incorporated into the vessel wall by tropoelastin secretion and the formation of an elastic lamina along their abluminal margin. After day 7, the new mural fibroblasts acquire the features of contractile cells, namely a basal lamina, extensive microfilaments, and dense bodies. In other vessels, as early as day 3 of hyperoxia, intermediate cells within the vessel intima begin to acquire the additional filaments and dense bodies of contractile cells. As hyperoxia continues, each cell pathway gives rise to vessels with distinct intimal or medial layers of contractile cells. In this way, thick-walled 'newly muscularized' vessel segments form adjacent to the capillary bed.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowden D. H., Adamson I. Y. Endothelial regeneration as a marker of the differential vascular responses in oxygen-induced pulmonary edema. Lab Invest. 1974 Mar;30(3):350–357. [PubMed] [Google Scholar]
- Clark J. G., Greenberg J. Modulation of the effects of alveolar macrophages on lung fibroblast collagen production rate. Am Rev Respir Dis. 1987 Jan;135(1):52–56. doi: 10.1164/arrd.1987.135.1.52. [DOI] [PubMed] [Google Scholar]
- Crapo J. D., Peters-Golden M., Marsh-Salin J., Shelburne J. S. Pathologic changes in the lungs of oxygen-adapted rats: a morphometric analysis. Lab Invest. 1978 Dec;39(6):640–653. [PubMed] [Google Scholar]
- Davies P., Burke G., Reid L. The structure of the wall of the rat intraacinar pulmonary artery: an electron microscopic study of microdissected preparations. Microvasc Res. 1986 Jul;32(1):50–63. doi: 10.1016/0026-2862(86)90043-9. [DOI] [PubMed] [Google Scholar]
- Eddy R. J., Petro J. A., Tomasek J. J. Evidence for the nonmuscle nature of the "myofibroblast" of granulation tissue and hypertropic scar. An immunofluorescence study. Am J Pathol. 1988 Feb;130(2):252–260. [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G., Hirschel B. J., Ryan G. B., Statkov P. R., Majno G. Granulation tissue as a contractile organ. A study of structure and function. J Exp Med. 1972 Apr 1;135(4):719–734. doi: 10.1084/jem.135.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hislop A., Reid L. Normal structure and dimensions of the pulmonary arteries in the rat. J Anat. 1978 Jan;125(Pt 1):71–83. [PMC free article] [PubMed] [Google Scholar]
- Hu L. M., Jones R. Injury and remodeling of pulmonary veins by high oxygen. A morphometric study. Am J Pathol. 1989 Feb;134(2):253–262. [PMC free article] [PubMed] [Google Scholar]
- Jester J. V., Rodrigues M. M., Herman I. M. Characterization of avascular corneal wound healing fibroblasts. New insights into the myofibroblast. Am J Pathol. 1987 Apr;127(1):140–148. [PMC free article] [PubMed] [Google Scholar]
- Jones R., Adler C., Farber F. Lung vascular cell proliferation in hyperoxic pulmonary hypertension and on return to air: [3H]thymidine pulse-labeling of intimal, medial, and adventitial cells in microvessels and at the hilum. Am Rev Respir Dis. 1989 Nov;140(5):1471–1477. doi: 10.1164/ajrccm/140.5.1471. [DOI] [PubMed] [Google Scholar]
- Jones R., Zapol W. M., Reid L. Oxygen toxicity and restructuring of pulmonary arteries--a morphometric study. The response to 4 weeks' exposure to hyperoxia and return to breathing air. Am J Pathol. 1985 Nov;121(2):212–223. [PMC free article] [PubMed] [Google Scholar]
- Jones R., Zapol W. M., Reid L. Pulmonary artery remodeling and pulmonary hypertension after exposure to hyperoxia for 7 days. A morphometric and hemodynamic study. Am J Pathol. 1984 Nov;117(2):273–285. [PMC free article] [PubMed] [Google Scholar]
- Kistler G. S., Caldwell P. R., Weibel E. R. Development of fine structural damage to alveolar and capillary lining cells in oxygen-poisoned rat lungs. J Cell Biol. 1967 Mar;32(3):605–628. doi: 10.1083/jcb.32.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecham R. P., Whitehouse L. A., Wrenn D. S., Parks W. C., Griffin G. L., Senior R. M., Crouch E. C., Stenmark K. R., Voelkel N. F. Smooth muscle-mediated connective tissue remodeling in pulmonary hypertension. Science. 1987 Jul 24;237(4813):423–426. doi: 10.1126/science.3603030. [DOI] [PubMed] [Google Scholar]
- Meyrick B., Reid L. The effect of continued hypoxia on rat pulmonary arterial circulation. An ultrastructural study. Lab Invest. 1978 Feb;38(2):188–200. [PubMed] [Google Scholar]
- Meyrick B., Reid L. Ultrastructural features of the distended pulmonary arteries of the normal rat. Anat Rec. 1979 Jan;193(1):71–97. doi: 10.1002/ar.1091930106. [DOI] [PubMed] [Google Scholar]
- Noguchi A., Reddy R., Kursar J. D., Parks W. C., Mecham R. P. Smooth muscle isoactin and elastin in fetal bovine lung. Exp Lung Res. 1989 Jul;15(4):537–552. doi: 10.3109/01902148909069617. [DOI] [PubMed] [Google Scholar]
- Sappino A. P., Schürch W., Gabbiani G. Differentiation repertoire of fibroblastic cells: expression of cytoskeletal proteins as marker of phenotypic modulations. Lab Invest. 1990 Aug;63(2):144–161. [PubMed] [Google Scholar]
- Schmidt R. A., Gown A. M. "Professional" and "nonprofessional" contractile cells in the lung. Am J Respir Cell Mol Biol. 1990 Dec;3(6):513–514. doi: 10.1165/ajrcmb/3.6.513. [DOI] [PubMed] [Google Scholar]
- Sims D. E. The pericyte--a review. Tissue Cell. 1986;18(2):153–174. doi: 10.1016/0040-8166(86)90026-1. [DOI] [PubMed] [Google Scholar]
- Smith P., Heath D. Ultrastructure of hypoxic hypertensive pulmonary vascular disease. J Pathol. 1977 Feb;121(2):93–100. doi: 10.1002/path.1711210205. [DOI] [PubMed] [Google Scholar]
- Sobin S. S., Tremer H. M., Hardy J. D., Chiodi H. P. Changes in arteriole in acute and chronic hypoxic pulmonary hypertension and recovery in rat. J Appl Physiol Respir Environ Exerc Physiol. 1983 Nov;55(5):1445–1455. doi: 10.1152/jappl.1983.55.5.1445. [DOI] [PubMed] [Google Scholar]
- Tomasek J. J., Hay E. D., Fujiwara K. Collagen modulates cell shape and cytoskeleton of embryonic corneal and fibroma fibroblasts: distribution of actin, alpha-actinin, and myosin. Dev Biol. 1982 Jul;92(1):107–122. doi: 10.1016/0012-1606(82)90155-5. [DOI] [PubMed] [Google Scholar]
- Vande Berg J. S., Rudolph R., Poolman W. L., Disharoon D. R. Comparative growth dynamics and actin concentration between cultured human myofibroblasts from granulating wounds and dermal fibroblasts from normal skin. Lab Invest. 1989 Nov;61(5):532–538. [PubMed] [Google Scholar]
- Weibel E. R. On pericytes, particularly their existence on lung capillaries. Microvasc Res. 1974 Sep;8(2):218–235. doi: 10.1016/0026-2862(74)90096-x. [DOI] [PubMed] [Google Scholar]