Abstract
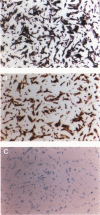
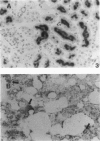
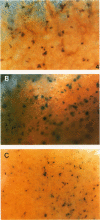
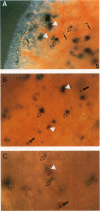
To evaluate interactions of two different tumor cell classes during the establishment of micrometastases at the single-cell level, two different BALB/c 3T3 tumor cell derivatives were established that harbor different histochemical marker genes: bacterial lacZ in a EJ-Harvey ras transformant (abbreviated LZEJ cells) and human placental alkaline phosphatase (ALP) gene in a human c-sis transformant (APSI cells). Several different histochemical staining methods were evaluated, using the distinctiveness of lacZ and ALP gene activities, for identification of these cell classes singly or together in the lung after their intravenous injection into nude mice. LZEJ and APSI cells could readily be distinguished from each other after co-injection by using specific and sequential staining protocols of whole organs or sections; staining of host organ cells was minimized. Co-injection of the two tumor cell classes resulted in similar numbers of homogeneous microfoci in lungs of LZEJ or APSI cells within minutes after injection that persisted for several hours before clearance of most of them. Furthermore, a significant percentage of foci could be identified containing both classes of tumor cells on whole-organ or section evaluations; these cohabiting foci resisted clearance from lungs. Therefore, use of two different histochemical marker genes to tag different classes of tumor cells provides a powerful approach for determining their in situ co-localization, cooperation, or interference with the establishment and development of micrometastases, as well as an opportunity to evaluate gene regulation in situ at the single-cell level.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barberá-Guillem E., Alonso-Varona A., Vidal-Vanaclocha F. Selective implantation and growth in rats and mice of experimental liver metastasis in acinar zone one. Cancer Res. 1989 Jul 15;49(14):4003–4010. [PubMed] [Google Scholar]
- Breillout F., Antoine E., Lascaux V., Rolland Y., Poupon M. F. Promotion of micrometastasis proliferation in a rat rhabdomyosarcoma model by epidermal growth factor. J Natl Cancer Inst. 1989 May 3;81(9):702–705. doi: 10.1093/jnci/81.9.702. [DOI] [PubMed] [Google Scholar]
- Cornil I., Theodorescu D., Man S., Herlyn M., Jambrosic J., Kerbel R. S. Fibroblast cell interactions with human melanoma cells affect tumor cell growth as a function of tumor progression. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6028–6032. doi: 10.1073/pnas.88.14.6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross M., Dexter T. M. Growth factors in development, transformation, and tumorigenesis. Cell. 1991 Jan 25;64(2):271–280. doi: 10.1016/0092-8674(91)90638-f. [DOI] [PubMed] [Google Scholar]
- Dikow A., Gossrau R., Frank H. G. Menadiol diphosphate, a new substrate for non-specific alkaline phosphatase in histochemistry and immunohistochemistry. Histochemistry. 1990;94(2):217–223. doi: 10.1007/BF02440191. [DOI] [PubMed] [Google Scholar]
- Doria M., Lloyd D., Thistlethwaite J. R., Franklin W. A. Immunohistochemical detection of antibody in tissue sections of non-perfused and ex vivo-perfused organs using a tetrazolium alkaline phosphatase substrate. Histochemistry. 1988;89(5):443–446. doi: 10.1007/BF00492600. [DOI] [PubMed] [Google Scholar]
- Egan S. E., Jarolim L., Rogelj S., Spearman M., Wright J. A., Greenberg A. H. Growth factor modulation of metastatic lung colonization. Anticancer Res. 1990 Sep-Oct;10(5A):1341–1346. [PubMed] [Google Scholar]
- Endo Y., Sasaki T., Harada F., Noguchi M. Specific detection of metastasized human tumor cells in embryonic chicks by the polymerase chain reaction. Jpn J Cancer Res. 1990 Aug;81(8):723–726. doi: 10.1111/j.1349-7006.1990.tb02635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eva A., Robbins K. C., Andersen P. R., Srinivasan A., Tronick S. R., Reddy E. P., Ellmore N. W., Galen A. T., Lautenberger J. A., Papas T. S. Cellular genes analogous to retroviral onc genes are transcribed in human tumour cells. Nature. 1982 Jan 14;295(5845):116–119. doi: 10.1038/295116a0. [DOI] [PubMed] [Google Scholar]
- Fidler I. J., Balch C. M. The biology of cancer metastasis and implications for therapy. Curr Probl Surg. 1987 Mar;24(3):129–209. doi: 10.1016/0011-3840(87)90002-5. [DOI] [PubMed] [Google Scholar]
- Fidler I. J. Critical factors in the biology of human cancer metastasis: twenty-eighth G.H.A. Clowes memorial award lecture. Cancer Res. 1990 Oct 1;50(19):6130–6138. [PubMed] [Google Scholar]
- Fidler I. J. Metastasis: quantitative analysis of distribution and fate of tumor emboli labeled with 125 I-5-iodo-2'-deoxyuridine. J Natl Cancer Inst. 1970 Oct;45(4):773–782. [PubMed] [Google Scholar]
- Fidler I. J. Selection of successive tumour lines for metastasis. Nat New Biol. 1973 Apr 4;242(118):148–149. doi: 10.1038/newbio242148a0. [DOI] [PubMed] [Google Scholar]
- Fidler I. J. The relationship of embolic homogeneity, number, size and viability to the incidence of experimental metastasis. Eur J Cancer. 1973 Mar;9(3):223–227. doi: 10.1016/s0014-2964(73)80022-2. [DOI] [PubMed] [Google Scholar]
- Fisher B., Fisher E. R. The organ distribution of disseminated 51 Cr-labeled tumor cells. Cancer Res. 1967 Feb;27(2):412–420. [PubMed] [Google Scholar]
- Frost P., Kerbel R. S., Hunt B., Man S., Pathak S. Selection of metastatic variants with identifiable karyotypic changes from a nonmetastatic murine tumor after treatment with 2'-deoxy-5-azacytidine or hydroxyurea: implications for the mechanisms of tumor progression. Cancer Res. 1987 May 15;47(10):2690–2695. [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Greig R. G., Trainer D. L. Shaping future strategies for the pharmacological control of tumor cell metastases. Cancer Metastasis Rev. 1986;5(1):3–14. doi: 10.1007/BF00049527. [DOI] [PubMed] [Google Scholar]
- Gudkov A. V., Kashkin K. N., Zaitsevskaya T. E., Troyanovsky S. M. Histo-blotting: hybridization in situ detection of specific RNAs on tissue sections transferred on nitrocellulose. Int J Cancer. 1989 Dec 15;44(6):1052–1056. doi: 10.1002/ijc.2910440619. [DOI] [PubMed] [Google Scholar]
- Hart I. R., Goode N. T., Wilson R. E. Molecular aspects of the metastatic cascade. Biochim Biophys Acta. 1989 Jul 28;989(1):65–84. doi: 10.1016/0304-419x(89)90035-8. [DOI] [PubMed] [Google Scholar]
- Henthorn P., Zervos P., Raducha M., Harris H., Kadesch T. Expression of a human placental alkaline phosphatase gene in transfected cells: use as a reporter for studies of gene expression. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6342–6346. doi: 10.1073/pnas.85.17.6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M., Fernandez B., Kerbel R. S. Highly pigmented human melanoma variant which metastasizes widely in nude mice, including to skin and brain. Cancer Res. 1988 Sep 1;48(17):4897–4903. [PubMed] [Google Scholar]
- Kerbel R. S., Waghorne C., Korczak B., Lagarde A., Breitman M. L. Clonal dominance of primary tumours by metastatic cells: genetic analysis and biological implications. Cancer Surv. 1988;7(4):597–629. [PubMed] [Google Scholar]
- Lin A. H., Groppi V. E., Gorman R. R. Platelet-derived growth factor does not induce c-fos in NIH 3T3 cells expressing the EJ-ras oncogene. Mol Cell Biol. 1988 Nov;8(11):5052–5055. doi: 10.1128/mcb.8.11.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W. C., Culp L. A. Selectable plasmid vectors with alternative and ultrasensitive histochemical marker genes. Biotechniques. 1991 Sep;11(3):344-8, 350-1. [PubMed] [Google Scholar]
- Lin W. C., Pretlow T. P., Pretlow T. G., 2nd, Culp L. A. Bacterial lacZ gene as a highly sensitive marker to detect micrometastasis formation during tumor progression. Cancer Res. 1990 May 1;50(9):2808–2817. [PubMed] [Google Scholar]
- Lin W. C., Pretlow T. P., Pretlow T. G., 3rd, Culp L. A. Development of micrometastases: earliest events detected with bacterial lacZ gene-tagged tumor cells. J Natl Cancer Inst. 1990 Sep 19;82(18):1497–1503. doi: 10.1093/jnci/82.18.1497. [DOI] [PubMed] [Google Scholar]
- McMorrow L. E., Wolman S. R., Bornstein S., Talmadge J. E. Irradiation-induced marker chromosomes in a metastasizing murine tumor. Cancer Res. 1988 Feb 15;48(4):999–1003. [PubMed] [Google Scholar]
- Miller F. R., Heppner G. H. Cellular interactions in metastasis. Cancer Metastasis Rev. 1990 Jul;9(1):21–34. doi: 10.1007/BF00047586. [DOI] [PubMed] [Google Scholar]
- Miller F. R., McInerney D. J., Rogers C., Aitken D. R., Wei W. Z. Use of drug resistance markers to recover clonogenic tumor cells from occult metastases in host tissues. Invasion Metastasis. 1986;6(4):197–208. [PubMed] [Google Scholar]
- Mottolese M., Venturo I., Digiesi G., Perrone Donnorso R., Bigotti A., Muraro R., Aluffi A., Natali P. G. Use of MoAb D612 in combination with a panel of MoAb for the immunocytochemical identification of metastases from colon-rectum carcinoma. Br J Cancer. 1990 Apr;61(4):626–630. doi: 10.1038/bjc.1990.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L. Cancer metastasis: tumor cell and host organ properties important in metastasis to specific secondary sites. Biochim Biophys Acta. 1988 Nov 15;948(2):175–224. doi: 10.1016/0304-419x(88)90010-8. [DOI] [PubMed] [Google Scholar]
- Potter K. M., Juacaba S. F., Price J. E., Tarin D. Observations on organ distribution of fluorescein-labelled tumour cells released intravascularly. Invasion Metastasis. 1983;3(4):221–233. [PubMed] [Google Scholar]
- Radinsky R., Culp L. A. Clonal dominance of select subsets of viral Kirsten ras(+)-transformed 3T3 cells during tumor progression. Int J Cancer. 1991 Apr 22;48(1):148–159. doi: 10.1002/ijc.2910480126. [DOI] [PubMed] [Google Scholar]
- Ro J., Bresser J., Ro J. Y., Brasfield F., Hortobagyi G., Blick M. SIS/PDGF-B expression in benign and malignant human breast lesions. Oncogene. 1989 Mar;4(3):351–354. [PubMed] [Google Scholar]
- Sanes J. R., Rubenstein J. L., Nicolas J. F. Use of a recombinant retrovirus to study post-implantation cell lineage in mouse embryos. EMBO J. 1986 Dec 1;5(12):3133–3142. doi: 10.1002/j.1460-2075.1986.tb04620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlimok G., Funke I., Holzmann B., Göttlinger G., Schmidt G., Häuser H., Swierkot S., Warnecke H. H., Schneider B., Koprowski H. Micrometastatic cancer cells in bone marrow: in vitro detection with anti-cytokeratin and in vivo labeling with anti-17-1A monoclonal antibodies. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8672–8676. doi: 10.1073/pnas.84.23.8672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge C., Tanio Y., Meeker A., Talmadge J., Zbar B. Tumor cells transfected with the neomycin resistance gene (neo) contain unique genetic markers useful for identification of tumor recurrence and metastasis. Invasion Metastasis. 1987;7(4):197–207. [PubMed] [Google Scholar]
- Tsuruo T., Watanabe M., Oh-hara T. Stimulation of the growth of metastatic clones of mouse colon adenocarcinoma 26 in vitro by platelet-derived growth factor. Jpn J Cancer Res. 1989 Feb;80(2):136–140. doi: 10.1111/j.1349-7006.1989.tb02281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD S., Jr Pathogenesis of metastasis formation observed in vivo in the rabbit ear chamber. AMA Arch Pathol. 1958 Oct;66(4):550–568. [PubMed] [Google Scholar]
- Westermark B., Heldin C. H. Platelet-derived growth factor in autocrine transformation. Cancer Res. 1991 Oct 1;51(19):5087–5092. [PubMed] [Google Scholar]
- Zullo J. N., Faller D. V. P21 v-ras inhibits induction of c-myc and c-fos expression by platelet-derived growth factor. Mol Cell Biol. 1988 Dec;8(12):5080–5085. doi: 10.1128/mcb.8.12.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]