Abstract
Fibronectins (FN) comprise a family of adhesive glycoproteins that are prominent components of wound healing. These proteins arise by alternative splicing of a single gene transcript at three sites, termed EIIIA, EIIIB, and V. Extravasated plasma FN, which lacks the EIIIA and EIIIB domains, along with fibrin, comprise the "provisional" matrix that forms within minutes of tissue injury. By 2 days after cutaneous excisional wounding in rats, total FN messenger RNA (mRNA) expression is increased locally and dramatically within the surrounding dermis, in the subjacent muscle (panniculus carnosus) and, notably, at the wound margins. Moreover, in contrast to normal skin, 2-day wounds express EIIIA- and EIIIB-containing "embryonic" FN mRNAs. To identify the cells responsible for synthesizing the various FN isoforms, we performed in situ hybridization with probes for the various FN mRNAs. Collagen and lysozyme probes were employed to distinguish fibroblasts from macrophages. At early intervals (2 days) after wounding, macrophages were the principal cells that expressed FN mRNA. Moreover, many of these cells expressed embryonic FN mRNAs. At 7 to 10 days, when the wound defect was maturing, fibroblasts were the major cells synthesizing these embryonic FNs. It is widely accepted that wound macrophages phagocytose debris and provide degradative enzymes and cytokines essential for early stages of tissue repair. Our findings suggest an additional function for wound macrophages--synthesis of embryonic FNs providing an extracellular matrix that facilitates wound repair, perhaps by promoting cell migration.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. C., Watt F. M. Changes in keratinocyte adhesion during terminal differentiation: reduction in fibronectin binding precedes alpha 5 beta 1 integrin loss from the cell surface. Cell. 1990 Oct 19;63(2):425–435. doi: 10.1016/0092-8674(90)90175-e. [DOI] [PubMed] [Google Scholar]
- Alitalo K., Hovi T., Vaheri A. Fibronectin is produced by human macrophages. J Exp Med. 1980 Mar 1;151(3):602–613. doi: 10.1084/jem.151.3.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assoian R. K., Fleurdelys B. E., Stevenson H. C., Miller P. J., Madtes D. K., Raines E. W., Ross R., Sporn M. B. Expression and secretion of type beta transforming growth factor by activated human macrophages. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6020–6024. doi: 10.1073/pnas.84.17.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsi L., Castellani P., Risso A. M., Leprini A., Zardi L. Transforming growth factor-beta regulates the splicing pattern of fibronectin messenger RNA precursor. FEBS Lett. 1990 Feb 12;261(1):175–178. doi: 10.1016/0014-5793(90)80664-5. [DOI] [PubMed] [Google Scholar]
- Broekelmann T. J., Limper A. H., Colby T. V., McDonald J. A. Transforming growth factor beta 1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6642–6646. doi: 10.1073/pnas.88.15.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chung L. P., Keshav S., Gordon S. Cloning the human lysozyme cDNA: inverted Alu repeat in the mRNA and in situ hybridization for macrophages and Paneth cells. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6227–6231. doi: 10.1073/pnas.85.17.6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A. Fibronectin matrix deposition and fibronectin receptor expression in healing and normal skin. J Invest Dermatol. 1990 Jun;94(6 Suppl):128S–134S. doi: 10.1111/1523-1747.ep12876104. [DOI] [PubMed] [Google Scholar]
- Cross M., Mangelsdorf I., Wedel A., Renkawitz R. Mouse lysozyme M gene: isolation, characterization, and expression studies. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6232–6236. doi: 10.1073/pnas.85.17.6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSimone D. W., Norton P. A., Hynes R. O. Identification and characterization of alternatively spliced fibronectin mRNAs expressed in early Xenopus embryos. Dev Biol. 1992 Feb;149(2):357–369. doi: 10.1016/0012-1606(92)90291-n. [DOI] [PubMed] [Google Scholar]
- Donaldson D. J., Mahan J. T., Smith G. N., Jr Newt epidermal cell migration in vitro and in vivo appears to involve Arg-Gly-Asp-Ser receptors. J Cell Sci. 1987 May;87(Pt 4):525–534. doi: 10.1242/jcs.87.4.525. [DOI] [PubMed] [Google Scholar]
- Ffrench-Constant C., Hynes R. O. Alternative splicing of fibronectin is temporally and spatially regulated in the chicken embryo. Development. 1989 Jun;106(2):375–388. doi: 10.1242/dev.106.2.375. [DOI] [PubMed] [Google Scholar]
- Ffrench-Constant C., Hynes R. O. Patterns of fibronectin gene expression and splicing during cell migration in chicken embryos. Development. 1988 Nov;104(3):369–382. doi: 10.1242/dev.104.3.369. [DOI] [PubMed] [Google Scholar]
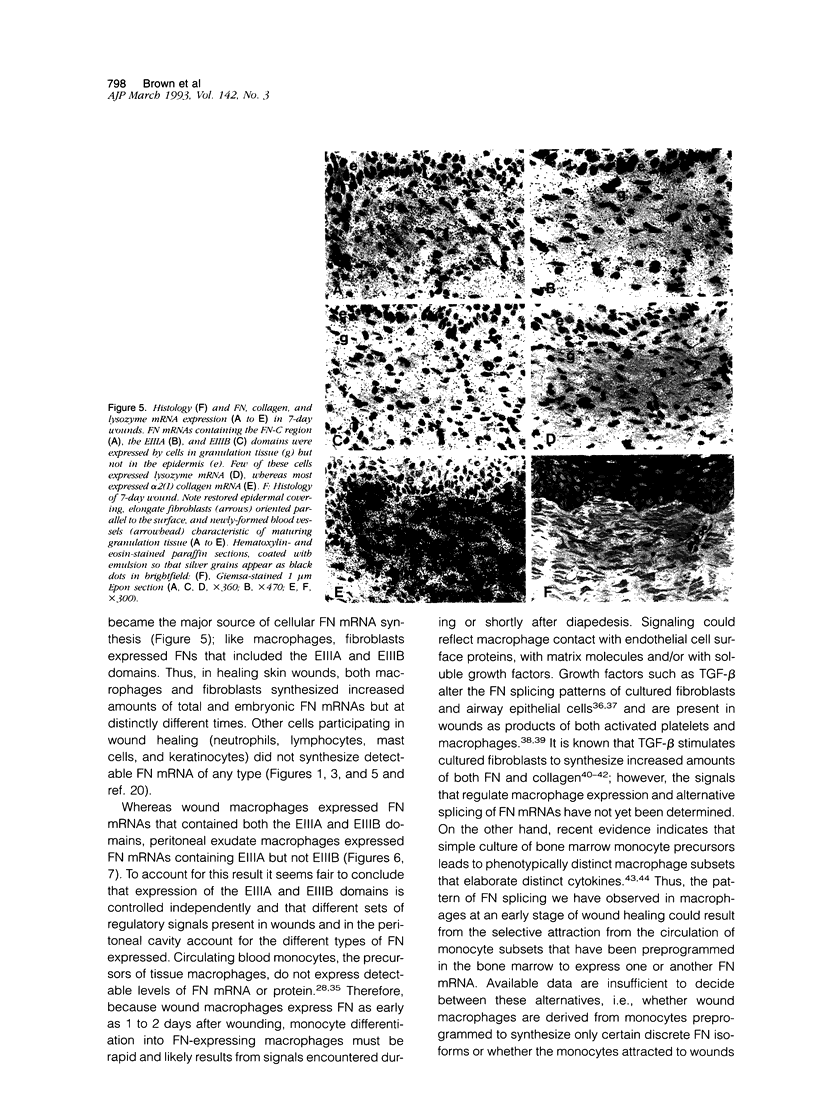
- Ffrench-Constant C., Van de Water L., Dvorak H. F., Hynes R. O. Reappearance of an embryonic pattern of fibronectin splicing during wound healing in the adult rat. J Cell Biol. 1989 Aug;109(2):903–914. doi: 10.1083/jcb.109.2.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell F. Fibronectin and wound healing. J Cell Biochem. 1984;26(2):107–116. doi: 10.1002/jcb.240260206. [DOI] [PubMed] [Google Scholar]
- Guo M., Toda K., Grinnell F. Activation of human keratinocyte migration on type I collagen and fibronectin. J Cell Sci. 1990 Jun;96(Pt 2):197–205. doi: 10.1242/jcs.96.2.197. [DOI] [PubMed] [Google Scholar]
- Humphries M. J., Komoriya A., Akiyama S. K., Olden K., Yamada K. M. Identification of two distinct regions of the type III connecting segment of human plasma fibronectin that promote cell type-specific adhesion. J Biol Chem. 1987 May 15;262(14):6886–6892. [PubMed] [Google Scholar]
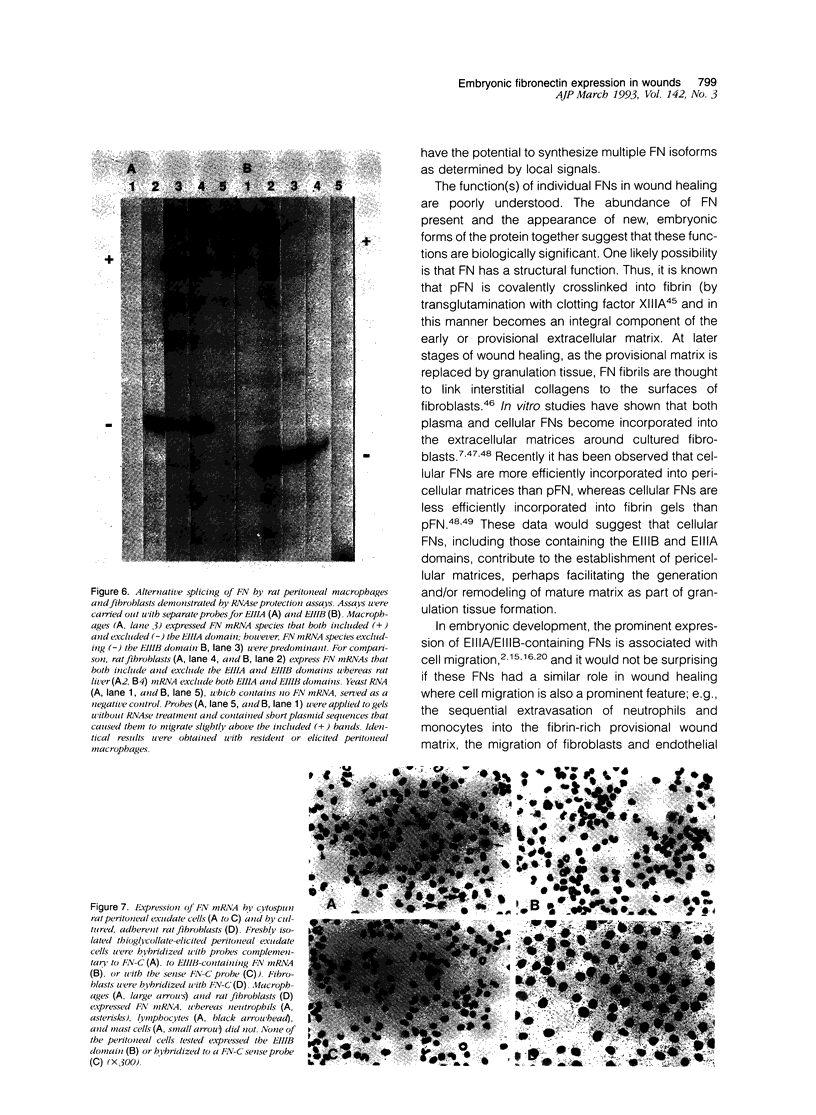
- Ignotz R. A., Endo T., Massagué J. Regulation of fibronectin and type I collagen mRNA levels by transforming growth factor-beta. J Biol Chem. 1987 May 15;262(14):6443–6446. [PubMed] [Google Scholar]
- Jaffe E. A., Ruggiero J. T., Falcone D. J. Monocytes and macrophages synthesize and secrete thrombospondin. Blood. 1985 Jan;65(1):79–84. [PubMed] [Google Scholar]
- Lindner V., Reidy M. A., Fingerle J. Regrowth of arterial endothelium. Denudation with minimal trauma leads to complete endothelial cell regrowth. Lab Invest. 1989 Nov;61(5):556–563. [PubMed] [Google Scholar]
- Madri J. A., Reidy M. A., Kocher O., Bell L. Endothelial cell behavior after denudation injury is modulated by transforming growth factor-beta1 and fibronectin. Lab Invest. 1989 Jun;60(6):755–765. [PubMed] [Google Scholar]
- Magnuson V. L., Young M., Schattenberg D. G., Mancini M. A., Chen D. L., Steffensen B., Klebe R. J. The alternative splicing of fibronectin pre-mRNA is altered during aging and in response to growth factors. J Biol Chem. 1991 Aug 5;266(22):14654–14662. [PubMed] [Google Scholar]
- McDonald J. A. Extracellular matrix assembly. Annu Rev Cell Biol. 1988;4:183–207. doi: 10.1146/annurev.cb.04.110188.001151. [DOI] [PubMed] [Google Scholar]
- Mosher D. F. Action of fibrin-stabilizing factor on cold-insoluble globulin and alpha2-macroglobulin in clotting plasma. J Biol Chem. 1976 Mar 25;251(6):1639–1645. [PubMed] [Google Scholar]
- Nathan C. F. Secretory products of macrophages. J Clin Invest. 1987 Feb;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C., Sporn M. Cytokines in context. J Cell Biol. 1991 Jun;113(5):981–986. doi: 10.1083/jcb.113.5.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton P. A., Hynes R. O. Alternative splicing of chicken fibronectin in embryos and in normal and transformed cells. Mol Cell Biol. 1987 Dec;7(12):4297–4307. doi: 10.1128/mcb.7.12.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani F., Zagato L., Vergani C., Casari G., Sidoli A., Baralle F. E. Tissue-specific splicing pattern of fibronectin messenger RNA precursor during development and aging in rat. J Cell Biol. 1991 Jun;113(5):1223–1229. doi: 10.1083/jcb.113.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters D. M., Portz L. M., Fullenwider J., Mosher D. F. Co-assembly of plasma and cellular fibronectins into fibrils in human fibroblast cultures. J Cell Biol. 1990 Jul;111(1):249–256. doi: 10.1083/jcb.111.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirami L., Stockinger B., Corradin S. B., Sironi M., Sassano M., Valsasnini P., Righi M., Ricciardi-Castagnoli P. Mouse macrophage clones immortalized by retroviruses are functionally heterogeneous. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7543–7547. doi: 10.1073/pnas.88.17.7543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghow R., Postlethwaite A. E., Keski-Oja J., Moses H. L., Kang A. H. Transforming growth factor-beta increases steady state levels of type I procollagen and fibronectin messenger RNAs posttranscriptionally in cultured human dermal fibroblasts. J Clin Invest. 1987 Apr;79(4):1285–1288. doi: 10.1172/JCI112950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappolee D. A., Werb Z. Secretory products of phagocytes. Curr Opin Immunol. 1988 Sep-Oct;1(1):47–55. doi: 10.1016/0952-7915(88)90050-7. [DOI] [PubMed] [Google Scholar]
- Roberts C. J., Birkenmeier T. M., McQuillan J. J., Akiyama S. K., Yamada S. S., Chen W. T., Yamada K. M., McDonald J. A. Transforming growth factor beta stimulates the expression of fibronectin and of both subunits of the human fibronectin receptor by cultured human lung fibroblasts. J Biol Chem. 1988 Apr 5;263(10):4586–4592. [PubMed] [Google Scholar]
- Ruoslahti E. Fibronectin and its receptors. Annu Rev Biochem. 1988;57:375–413. doi: 10.1146/annurev.bi.57.070188.002111. [DOI] [PubMed] [Google Scholar]
- Schwarzbauer J. E. Alternative splicing of fibronectin: three variants, three functions. Bioessays. 1991 Oct;13(10):527–533. doi: 10.1002/bies.950131006. [DOI] [PubMed] [Google Scholar]
- Schwarzbauer J. E., Patel R. S., Fonda D., Hynes R. O. Multiple sites of alternative splicing of the rat fibronectin gene transcript. EMBO J. 1987 Sep;6(9):2573–2580. doi: 10.1002/j.1460-2075.1987.tb02547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senger D. R., Perruzzi C. A., Gracey C. F., Papadopoulos A., Tenen D. G. Secreted phosphoproteins associated with neoplastic transformation: close homology with plasma proteins cleaved during blood coagulation. Cancer Res. 1988 Oct 15;48(20):5770–5774. [PubMed] [Google Scholar]
- Singer I. I., Kawka D. W., Kazazis D. M., Clark R. A. In vivo co-distribution of fibronectin and actin fibers in granulation tissue: immunofluorescence and electron microscope studies of the fibronexus at the myofibroblast surface. J Cell Biol. 1984 Jun;98(6):2091–2106. doi: 10.1083/jcb.98.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villiger B., Kelley D. G., Engleman W., Kuhn C., 3rd, McDonald J. A. Human alveolar macrophage fibronectin: synthesis, secretion, and ultrastructural localization during gelatin-coated latex particle binding. J Cell Biol. 1981 Sep;90(3):711–720. doi: 10.1083/jcb.90.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A., Cohen D. S., Palmer E., Sheppard D. Polarized regulation of fibronectin secretion and alternative splicing by transforming growth factor. J Biol Chem. 1991 Aug 25;266(24):15598–15601. [PubMed] [Google Scholar]
- Wilson C. L., Schwarzbauer J. E. The alternatively spliced V region contributes to the differential incorporation of plasma and cellular fibronectins into fibrin clots. J Cell Biol. 1992 Nov;119(4):923–933. doi: 10.1083/jcb.119.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witsell A. L., Schook L. B. Macrophage heterogeneity occurs through a developmental mechanism. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1963–1967. doi: 10.1073/pnas.88.5.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M. Fibronectins: structure, functions and receptors. Curr Opin Cell Biol. 1989 Oct;1(5):956–963. doi: 10.1016/0955-0674(89)90065-3. [DOI] [PubMed] [Google Scholar]
- Yamauchi K., Martinet Y., Crystal R. G. Modulation of fibronectin gene expression in human mononuclear phagocytes. J Clin Invest. 1987 Dec;80(6):1720–1727. doi: 10.1172/JCI113263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Water L., 3rd, Schroeder S., Crenshaw E. B., 3rd, Hynes R. O. Phagocytosis of gelatin-latex particles by a murine macrophage line is dependent on fibronectin and heparin. J Cell Biol. 1981 Jul;90(1):32–39. doi: 10.1083/jcb.90.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]