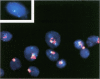
Abstract
Karyotype information on ovarian carcinomas has been limited because the tumors are often difficult to culture and the resultant metaphases can have complex numerical and structural chromosomal anomalies. Fluorescent in situ hybridization is a rapid method of determining centromere copy number in metaphase cells and interphase nuclei. Fluorescent in situ hybridization was used to determine the numerical centromere complement of chromosomes X, 8, 12, and 17 and HER-2/neu gene amplification within interphase nuclei of 25 primary epithelial ovarian carcinomas. Touch preparations of the carcinomas were hybridized with two-color combinations of directly labeled alpha-satellite centromeric chromosome enumeration probes and a directly labeled HER-2/neu probe. Modal centromere copy numbers for each of the four chromosomes were used to determine numerical abnormalities relative to the flow cytometric DNA ploidy level for each tumor. Four cases were found to be normal with respect to the four chromosomes studied. In the remaining 21 cases a relative loss of chromosomes 17 (16 cases) and X (nine cases) and a relative gain of chromosomes 12 (10 cases) and 8 (nine cases) were the most common findings. In addition, the HER-2/neu gene was amplified in two of the 25 tumors. In conclusion, fluorescent in situ hybridization is an excellent method for rapid determination of numerical abnormalities and gene amplification in ovarian carcinomas.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkin N. B., Baker M. C. Abnormal chromosomes including small metacentrics in 14 ovarian cancers. Cancer Genet Cytogenet. 1987 Jun;26(2):355–361. doi: 10.1016/0165-4608(87)90070-7. [DOI] [PubMed] [Google Scholar]
- Berchuck A., Kamel A., Whitaker R., Kerns B., Olt G., Kinney R., Soper J. T., Dodge R., Clarke-Pearson D. L., Marks P. Overexpression of HER-2/neu is associated with poor survival in advanced epithelial ovarian cancer. Cancer Res. 1990 Jul 1;50(13):4087–4091. [PubMed] [Google Scholar]
- Boltz E. M., Harnett P., Leary J., Houghton R., Kefford R. F., Friedlander M. L. Demonstration of somatic rearrangements and genomic heterogeneity in human ovarian cancer by DNA fingerprinting. Br J Cancer. 1990 Jul;62(1):23–27. doi: 10.1038/bjc.1990.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boring C. C., Squires T. S., Tong T. Cancer statistics, 1992. CA Cancer J Clin. 1992 Jan-Feb;42(1):19–38. doi: 10.3322/canjclin.42.1.19. [DOI] [PubMed] [Google Scholar]
- Eccles D. M., Cranston G., Steel C. M., Nakamura Y., Leonard R. C. Allele losses on chromosome 17 in human epithelial ovarian carcinoma. Oncogene. 1990 Oct;5(10):1599–1601. [PubMed] [Google Scholar]
- Gonchoroff N. J., Ryan J. J., Kimlinger T. K., Witzig T. E., Greipp P. R., Meyer J. S., Katzmann J. A. Effect of sonication on paraffin-embedded tissue preparation for DNA flow cytometry. Cytometry. 1990;11(5):642–646. doi: 10.1002/cyto.990110513. [DOI] [PubMed] [Google Scholar]
- Hedley D. W., Friedlander M. L., Taylor I. W., Rugg C. A., Musgrove E. A. Method for analysis of cellular DNA content of paraffin-embedded pathological material using flow cytometry. J Histochem Cytochem. 1983 Nov;31(11):1333–1335. doi: 10.1177/31.11.6619538. [DOI] [PubMed] [Google Scholar]
- Huber H., Knogler W., Karlic H., Akrad M., Söregi G., Schweizer D. Structural chromosomal abnormalities in gynecologic malignancies. Cancer Genet Cytogenet. 1990 Dec;50(2):189–197. doi: 10.1016/0165-4608(90)90179-e. [DOI] [PubMed] [Google Scholar]
- Kiechle-Schwarz M., Pfleiderer A., Sreekantaiah C., Berger C. S., Medchill M. T., Sandberg A. A. Cluster of trisomy 12 to tumors of the female genitourinary tract. Cancer Genet Cytogenet. 1991 Jul 15;54(2):273–275. doi: 10.1016/0165-4608(91)90223-h. [DOI] [PubMed] [Google Scholar]
- Kiechle-Schwarz M., Sreekantaiah C., Berger C. S., Pedron S., Medchill M. T., Surti U., Sandberg A. A. Nonrandom cytogenetic changes in leiomyomas of the female genitourinary tract. A report of 35 cases. Cancer Genet Cytogenet. 1991 May;53(1):125–136. doi: 10.1016/0165-4608(91)90124-d. [DOI] [PubMed] [Google Scholar]
- King B. L., Carter D., Foellmer H. G., Kacinski B. M. Neu proto-oncogene amplification and expression in ovarian adenocarcinoma cell lines. Am J Pathol. 1992 Jan;140(1):23–31. [PMC free article] [PubMed] [Google Scholar]
- Kusyk C. J., Turpening E. L., Edwards C. L., Wharton J. T., Copeland L. J. Karyotype analysis of four solid gynecologic tumors. Gynecol Oncol. 1982 Dec;14(3):324–338. doi: 10.1016/0090-8258(82)90107-x. [DOI] [PubMed] [Google Scholar]
- Lee J. H., Kavanagh J. J., Wildrick D. M., Wharton J. T., Blick M. Frequent loss of heterozygosity on chromosomes 6q, 11, and 17 in human ovarian carcinomas. Cancer Res. 1990 May 1;50(9):2724–2728. [PubMed] [Google Scholar]
- Leung W. Y., Schwartz P. E., Ng H. T., Yang-Feng T. L. Trisomy 12 in benign fibroma and granulosa cell tumor of the ovary. Gynecol Oncol. 1990 Jul;38(1):28–31. doi: 10.1016/0090-8258(90)90006-7. [DOI] [PubMed] [Google Scholar]
- Masuda H., Battifora H., Yokota J., Meltzer S., Cline M. J. Specificity of proto-oncogene amplification in human malignant diseases. Mol Biol Med. 1987 Aug;4(4):213–227. [PubMed] [Google Scholar]
- Mrózek K., Nedoszytko B., Babińska M., Mrózek E., Hrabowska M., Emerich J., Limon J. Trisomy of chromosome 12 in a case of thecoma of the ovary. Gynecol Oncol. 1990 Mar;36(3):413–416. doi: 10.1016/0090-8258(90)90154-d. [DOI] [PubMed] [Google Scholar]
- Nilbert M., Heim S., Mandahl N., Flodérus U. M., Willén H., Mitelman F. Trisomy 12 in uterine leiomyomas. A new cytogenetic subgroup. Cancer Genet Cytogenet. 1990 Mar;45(1):63–66. doi: 10.1016/0165-4608(90)90067-k. [DOI] [PubMed] [Google Scholar]
- Okamoto A., Sameshima Y., Yokoyama S., Terashima Y., Sugimura T., Terada M., Yokota J. Frequent allelic losses and mutations of the p53 gene in human ovarian cancer. Cancer Res. 1991 Oct 1;51(19):5171–5176. [PubMed] [Google Scholar]
- Pejovic T., Heim S., Mandahl N., Baldetorp B., Elmfors B., Flodérus U. M., Furgyik S., Helm G., Himmelmann A., Willén H. Chromosome aberrations in 35 primary ovarian carcinomas. Genes Chromosomes Cancer. 1992 Jan;4(1):58–68. doi: 10.1002/gcc.2870040108. [DOI] [PubMed] [Google Scholar]
- Pejovic T., Heim S., Mandahl N., Elmfors B., Flodérus U. M., Furgyik S., Helm G., Willén H., Mitelman F. Consistent occurrence of a 19p+ marker chromosome and loss of 11p material in ovarian seropapillary cystadenocarcinomas. Genes Chromosomes Cancer. 1989 Nov;1(2):167–171. doi: 10.1002/gcc.2870010210. [DOI] [PubMed] [Google Scholar]
- Pejovic T., Heim S., Mandahl N., Elmfors B., Flodérus U. M., Furgyik S., Helm G., Willén H., Mitelman F. Trisomy 12 is a consistent chromosomal aberration in benign ovarian tumors. Genes Chromosomes Cancer. 1990 May;2(1):48–52. doi: 10.1002/gcc.2870020109. [DOI] [PubMed] [Google Scholar]
- Pejovic T., Heim S., Mandahl N., Elmfors B., Furgyik S., Flodérus U. M., Helm G., Willén H., Mitelman F. Bilateral ovarian carcinoma: cytogenetic evidence of unicentric origin. Int J Cancer. 1991 Feb 1;47(3):358–361. doi: 10.1002/ijc.2910470308. [DOI] [PubMed] [Google Scholar]
- Pinkel D., Straume T., Gray J. W. Cytogenetic analysis using quantitative, high-sensitivity, fluorescence hybridization. Proc Natl Acad Sci U S A. 1986 May;83(9):2934–2938. doi: 10.1073/pnas.83.9.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts C. G., Tattersall M. H. Cytogenetic study of solid ovarian tumors. Cancer Genet Cytogenet. 1990 Sep;48(2):243–253. doi: 10.1016/0165-4608(90)90127-v. [DOI] [PubMed] [Google Scholar]
- Sato T., Saito H., Morita R., Koi S., Lee J. H., Nakamura Y. Allelotype of human ovarian cancer. Cancer Res. 1991 Oct 1;51(19):5118–5122. [PubMed] [Google Scholar]
- Sheer D., Sheppard D. M., Gorman P. A., Ward B., Whelan R. D., Hill B. T. Cytogenetic analysis of four human ovarian carcinoma cell lines. Cancer Genet Cytogenet. 1987 Jun;26(2):339–349. doi: 10.1016/0165-4608(87)90068-9. [DOI] [PubMed] [Google Scholar]
- Slamon D. J., Godolphin W., Jones L. A., Holt J. A., Wong S. G., Keith D. E., Levin W. J., Stuart S. G., Udove J., Ullrich A. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989 May 12;244(4905):707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- Smith A., Roberts C., van Haaften-Day C., den Dulk G., Russell P., Tattersall M. H. Cytogenetic findings in cell lines derived from four ovarian carcinomas. Cancer Genet Cytogenet. 1987 Feb;24(2):231–242. doi: 10.1016/0165-4608(87)90104-x. [DOI] [PubMed] [Google Scholar]
- Tyson F. L., Boyer C. M., Kaufman R., O'Briant K., Cram G., Crews J. R., Soper J. T., Daly L., Fowler W. C., Jr, Haskill J. S. Expression and amplification of the HER-2/neu (c-erbB-2) protooncogene in epithelial ovarian tumors and cell lines. Am J Obstet Gynecol. 1991 Sep;165(3):640–646. doi: 10.1016/0002-9378(91)90300-g. [DOI] [PubMed] [Google Scholar]
- Vanni R., Nieddu M., Paoli R., Lecca U. Uterine leiomyoma cytogenetics. I. Rearrangements of chromosome 12. Cancer Genet Cytogenet. 1989 Jan;37(1):49–54. doi: 10.1016/0165-4608(89)90073-3. [DOI] [PubMed] [Google Scholar]
- Vindeløv L. L., Christensen I. J., Nissen N. I. A detergent-trypsin method for the preparation of nuclei for flow cytometric DNA analysis. Cytometry. 1983 Mar;3(5):323–327. doi: 10.1002/cyto.990030503. [DOI] [PubMed] [Google Scholar]
- Yang-Feng T. L., Li S. B., Leung W. Y., Carcangiu M. L., Schwartz P. E. Trisomy 12 and K-ras-2 amplification in human ovarian tumors. Int J Cancer. 1991 Jul 9;48(5):678–681. doi: 10.1002/ijc.2910480508. [DOI] [PubMed] [Google Scholar]
- Zheng J. P., Robinson W. R., Ehlen T., Yu M. C., Dubeau L. Distinction of low grade from high grade human ovarian carcinomas on the basis of losses of heterozygosity on chromosomes 3, 6, and 11 and HER-2/neu gene amplification. Cancer Res. 1991 Aug 1;51(15):4045–4051. [PubMed] [Google Scholar]