Abstract
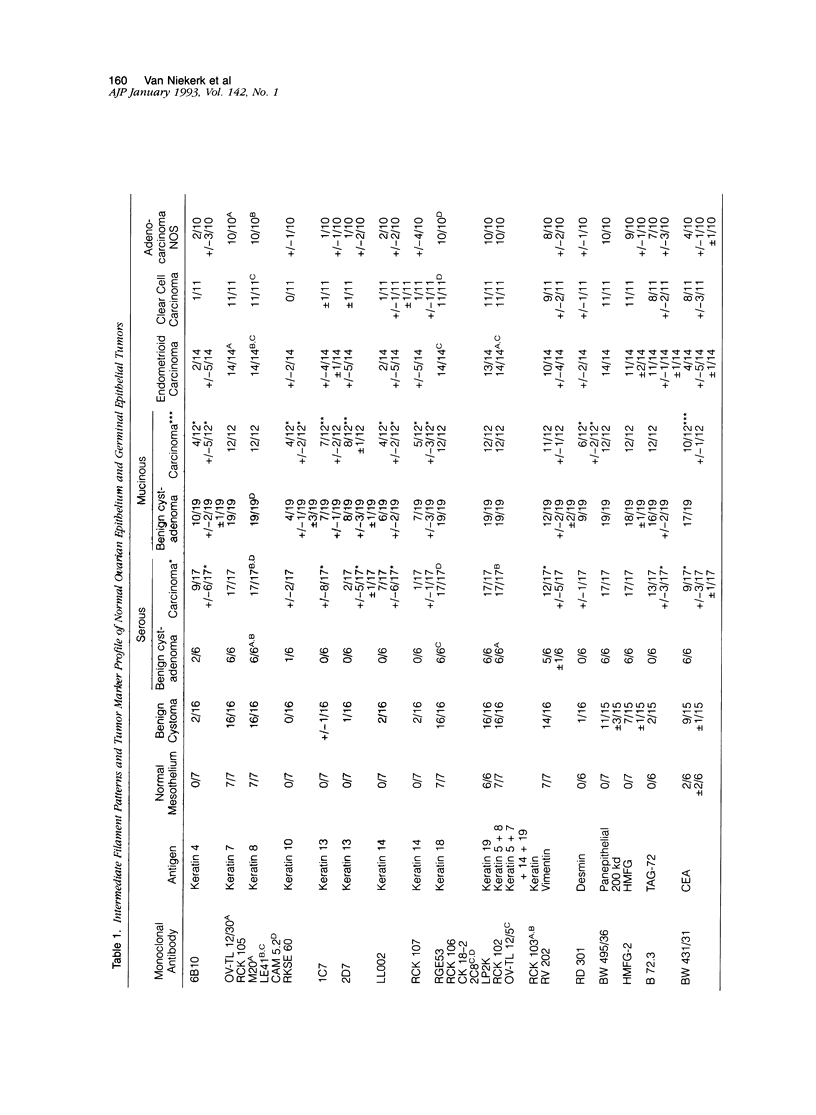
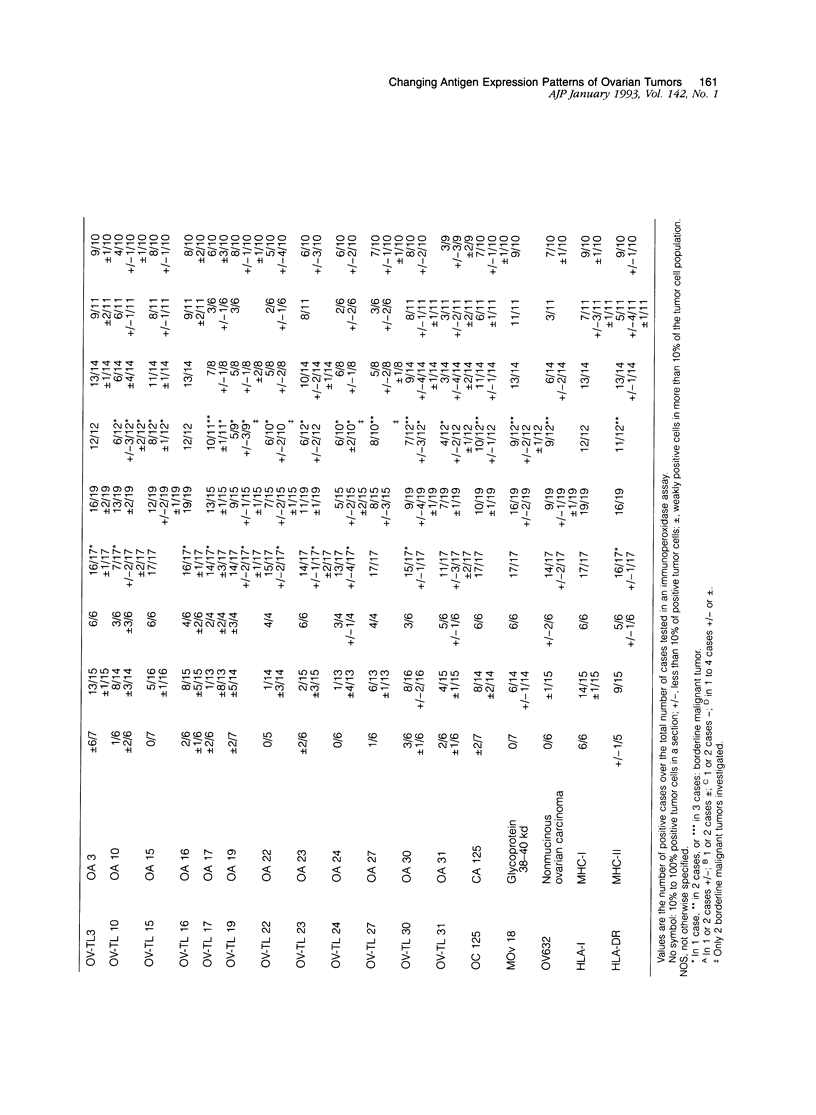
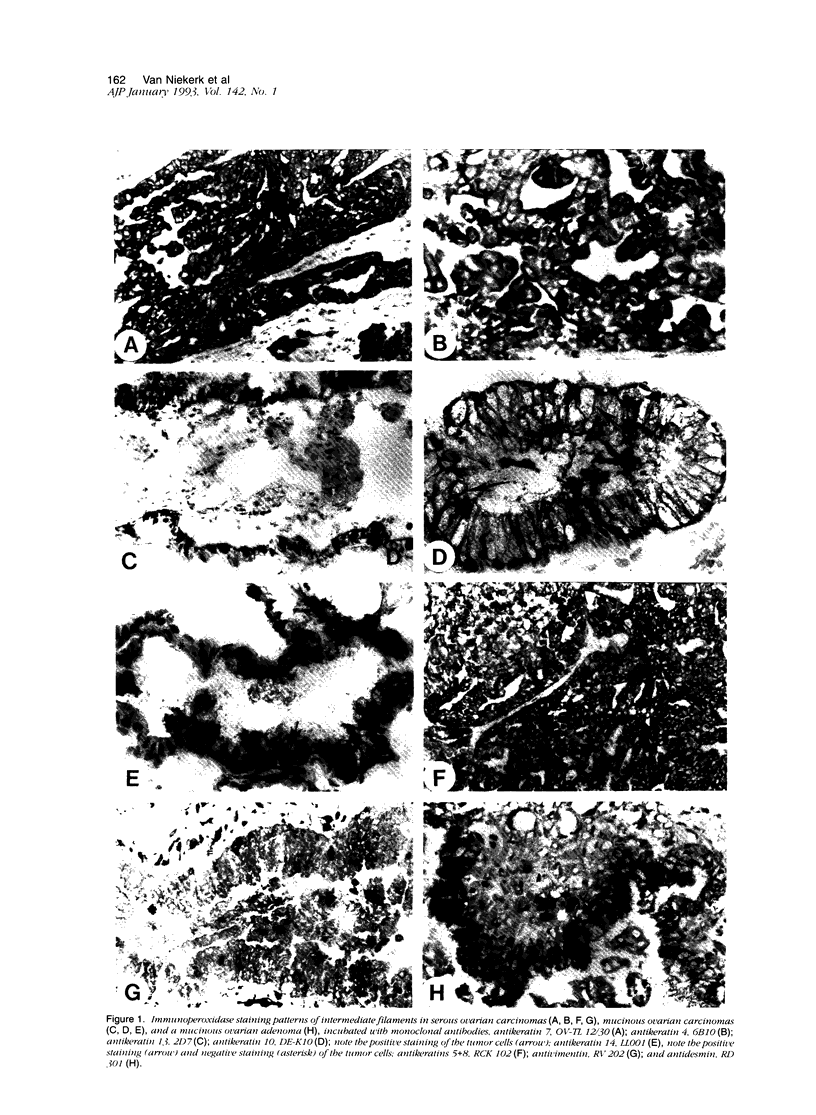
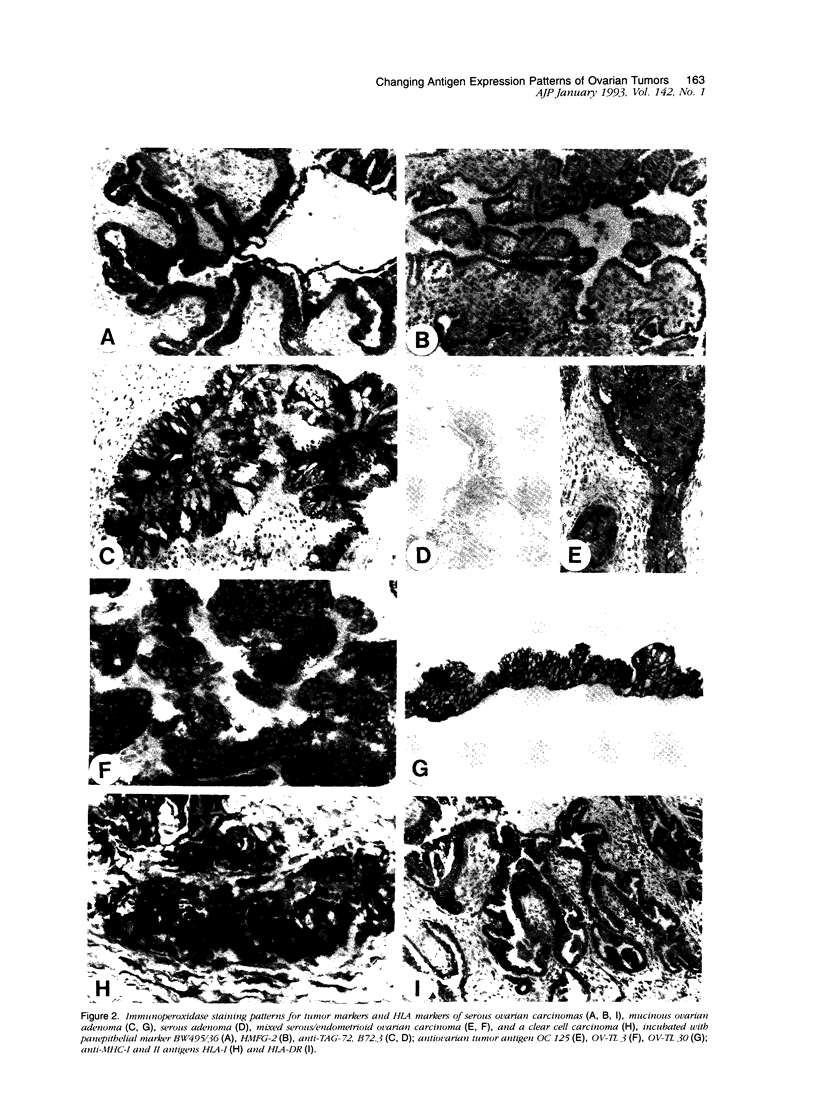
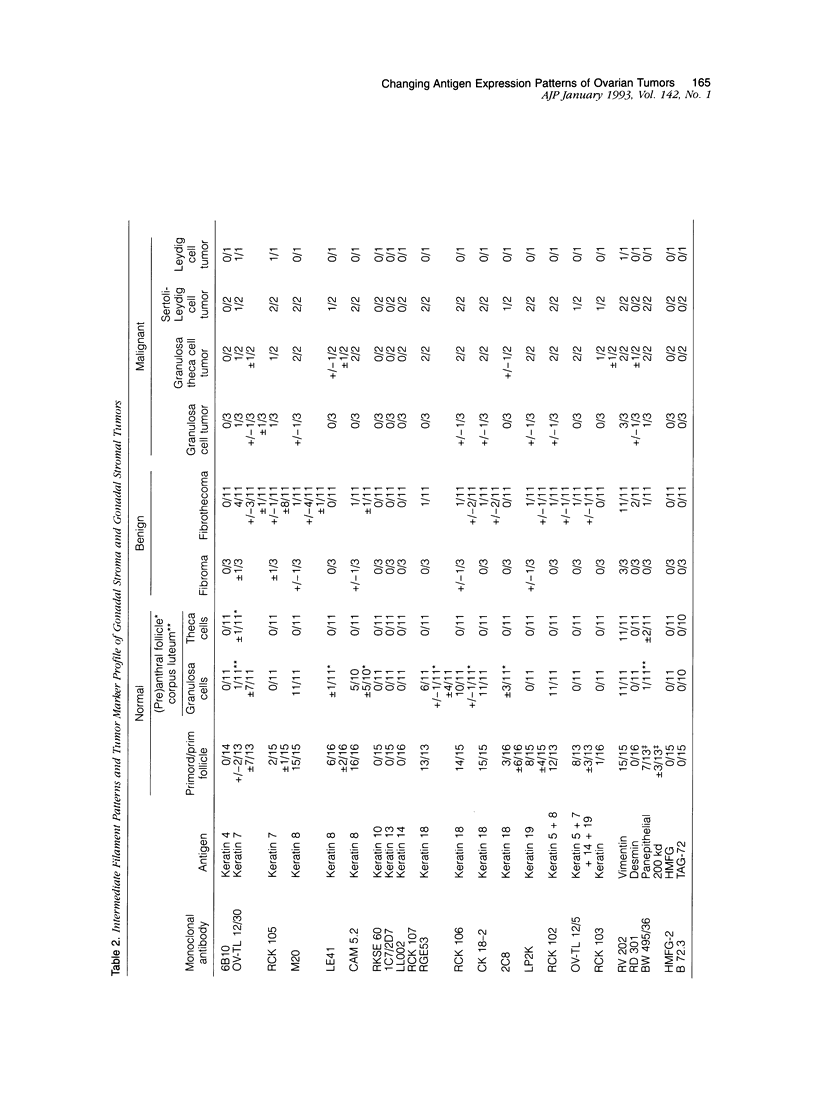
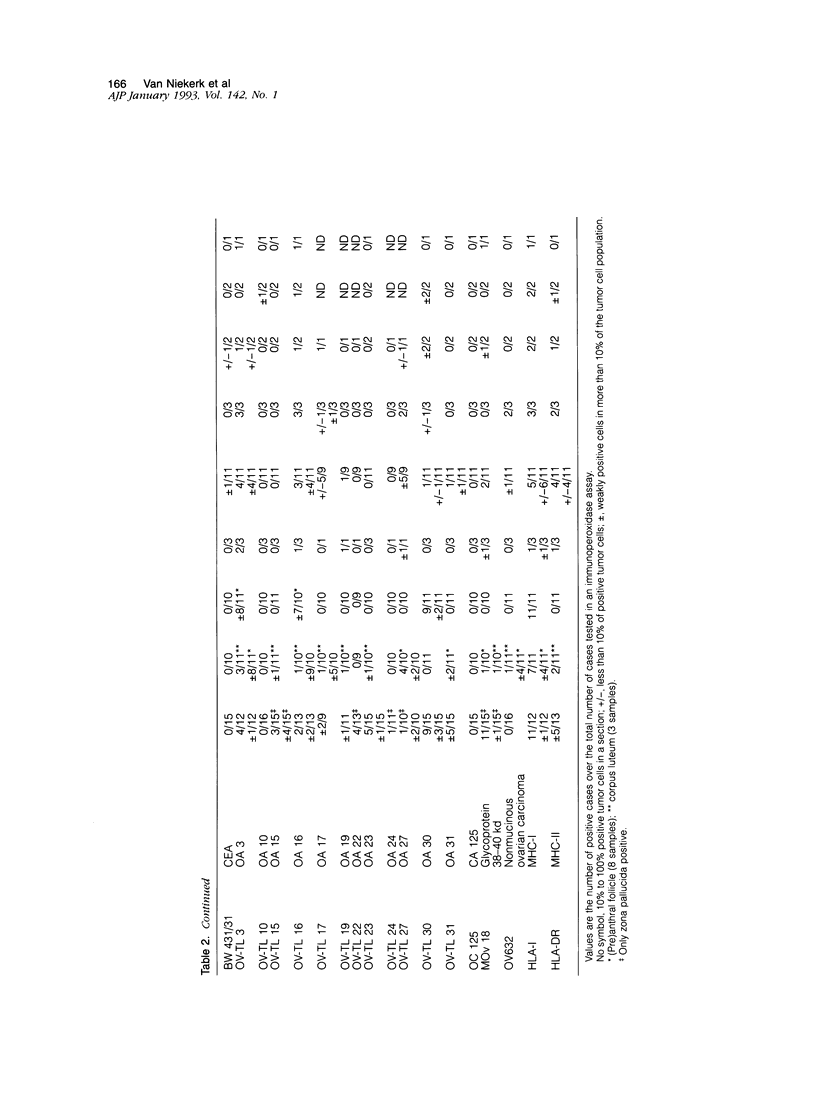
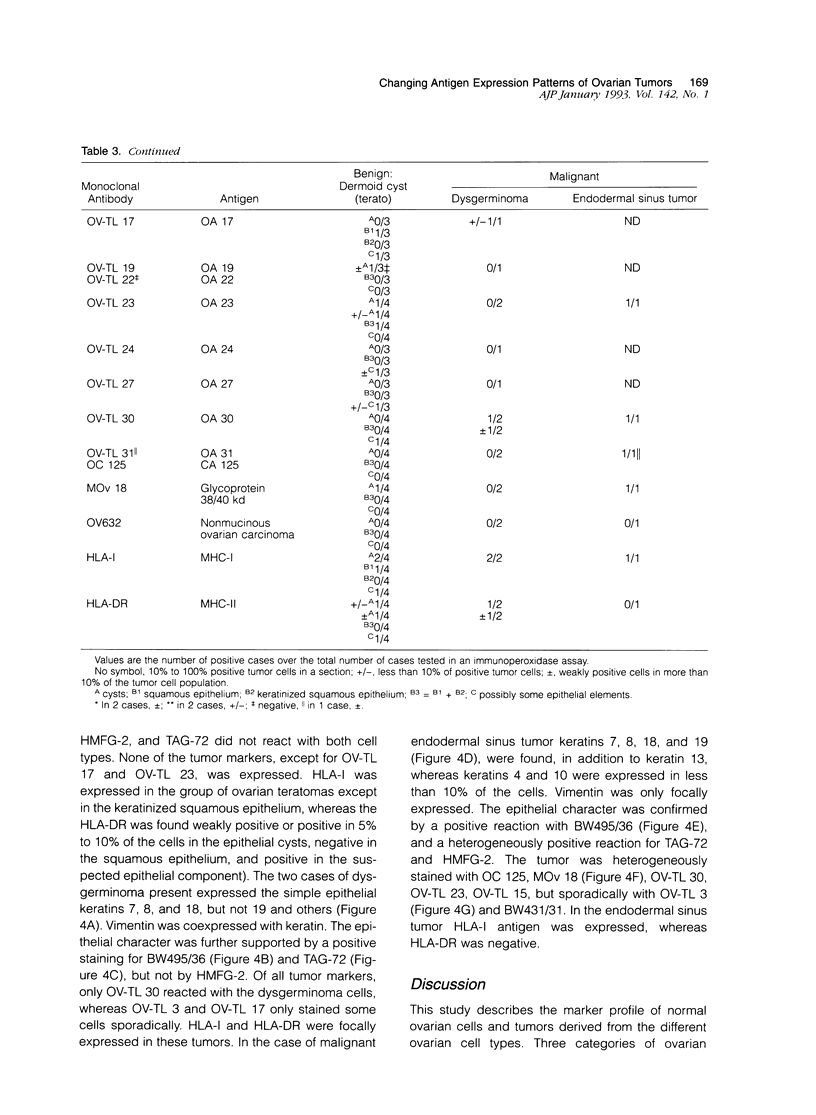
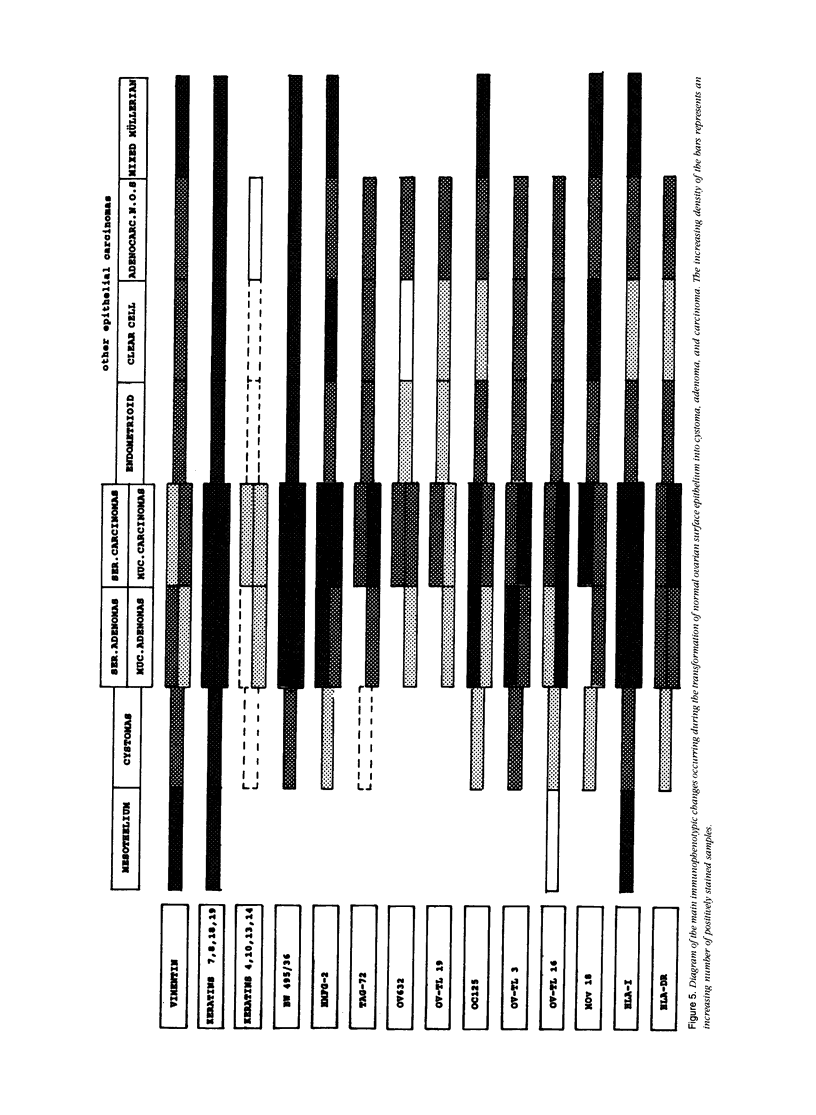
The marker profile of 18 samples of normal human ovarian tissues and 138 samples of their derived tumors was established using 51 monoclonal antibodies directed against intermediate filaments, ovarian carcinoma-specific antigens, general tumor-associated antigens and MHC-I/II antigens. Our data show that vimentin and keratins 7, 8, 18, and 19 were found in both epithelial and some nonepithelial ovarian tumors. Several tumor samples contained additional keratins 4, 10, 13, and 14, as well as desmin. BW 495/36 and to a lesser extent HMFG-2 were usually found in all ovarian tumors that contained simple epithelial keratins, except the absence of HMFG-2 in gonadal tumors as well as in dysgerminomas. In contrast to the keratin antibodies, these two panepithelial antibodies were negative in normal mesothelial cells and granulosa cells of the ovarian follicles. In general, the marker TAG-72 appeared useful for its discrimination between positively stained mucinous adenomas, the ovarian carcinomas as well as germ cell tumors, and the negatively stained gonadal tumors, serous adenomas, and cystomas. OV632 appeared useful in the distinction between negatively stained serous adenomas and positively stained serous carcinomas. In contrast, the monoclonal antibodies OC 125, OV-TL 3, OV-TL 16, and MOv 18 can be considered as pan-ovarian carcinoma markers, however without the discriminative capability as seen for OV632. These ovarian carcinoma-associated antigens were hardly found expressed in gonadal and germ cell tumors, except in the group of endodermal sinus tumors. HLA-I was found to be expressed in almost all nucleated cells, although loss of HLA-I expression was seen in areas of tumor cells. HLA-DR was negative in normal ovarian tissue, but heterogeneous expression was noticed in most of the epithelial tumors.
Full text
PDF



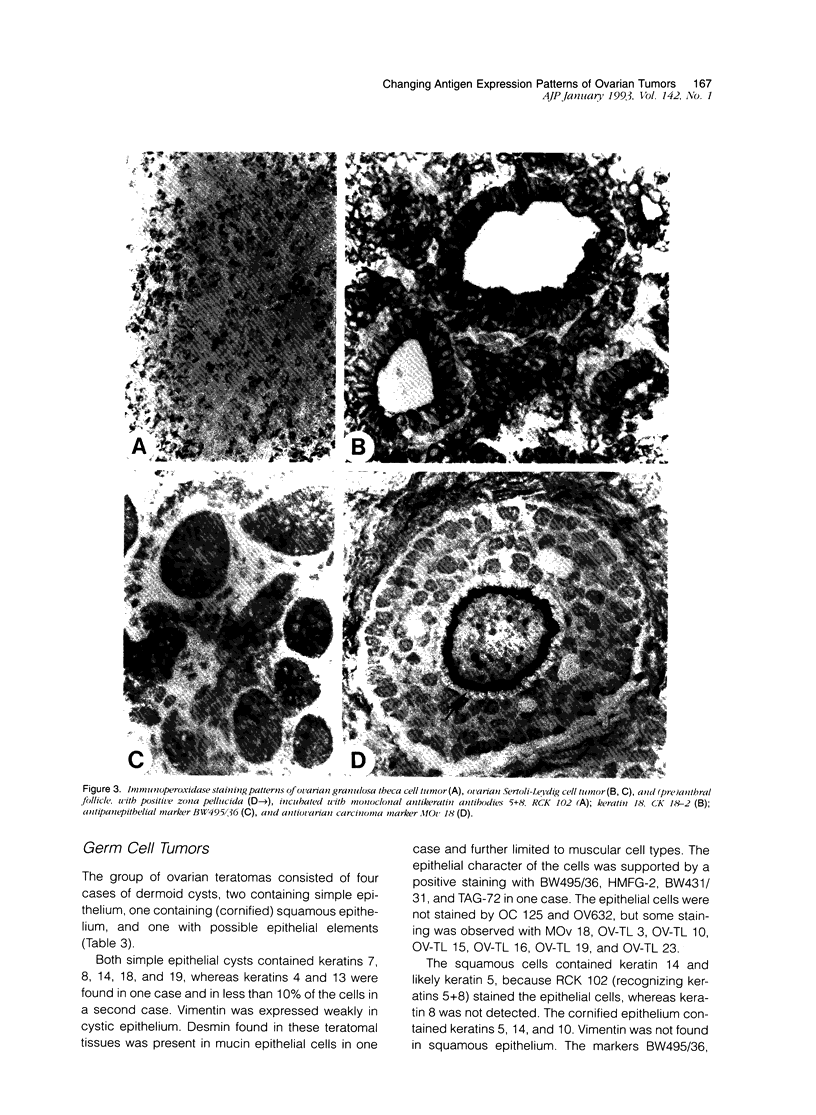
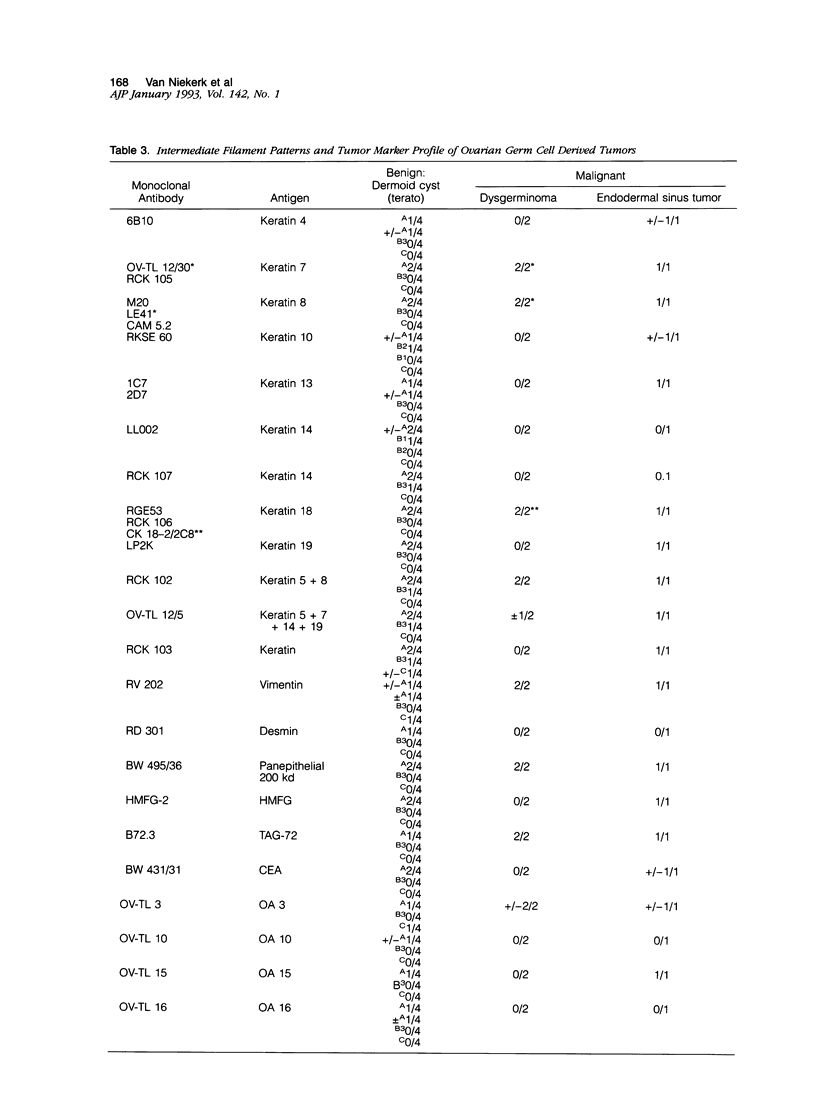






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bast R. C., Jr, Feeney M., Lazarus H., Nadler L. M., Colvin R. B., Knapp R. C. Reactivity of a monoclonal antibody with human ovarian carcinoma. J Clin Invest. 1981 Nov;68(5):1331–1337. doi: 10.1172/JCI110380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Amsterdam A. Regulation of cytoskeletal protein organization and expression in human granulosa cells in response to gonadotropin treatment. Endocrinology. 1989 Feb;124(2):1033–1041. doi: 10.1210/endo-124-2-1033. [DOI] [PubMed] [Google Scholar]
- Benjamin E., Law S., Bobrow L. G. Intermediate filaments cytokeratin and vimentin in ovarian sex cord-stromal tumours with correlative studies in adult and fetal ovaries. J Pathol. 1987 Aug;152(4):253–263. doi: 10.1002/path.1711520403. [DOI] [PubMed] [Google Scholar]
- Boerman O. C., Makkink W. K., Thomas C. M., Hanselaar A. G., Yedema C. A., Kenemans P., Poels L. G. Monoclonal antibodies that discriminate between human ovarian carcinomas and benign ovarian tumours. Eur J Cancer. 1990 Feb;26(2):117–127. doi: 10.1016/0277-5379(90)90293-3. [DOI] [PubMed] [Google Scholar]
- Boerman O. C., van Niekerk C. C., Makkink K., Hanselaar T. G., Kenemans P., Poels L. G. Comparative immunohistochemical study of four monoclonal antibodies directed against ovarian carcinoma-associated antigens. Int J Gynecol Pathol. 1991;10(1):15–25. doi: 10.1097/00004347-199101000-00002. [DOI] [PubMed] [Google Scholar]
- Boerman O., Makkink K., Massuger L., Thomas C., Kenemans P., Hanselaar T., Poels L. Monoclonal antibodies against ovarian carcinoma-associated antigens, raised by immunization with cyst fluids. Anticancer Res. 1989 May-Jun;9(3):551–558. [PubMed] [Google Scholar]
- Bosslet K., Lüben G., Schwarz A., Hundt E., Harthus H. P., Seiler F. R., Muhrer C., Klöppel G., Kayser K., Sedlacek H. H. Immunohistochemical localization and molecular characteristics of three monoclonal antibody-defined epitopes detectable on carcinoembryonic antigen (CEA). Int J Cancer. 1985 Jul 15;36(1):75–84. doi: 10.1002/ijc.2910360113. [DOI] [PubMed] [Google Scholar]
- Campbell I. G., Jones T. A., Foulkes W. D., Trowsdale J. Folate-binding protein is a marker for ovarian cancer. Cancer Res. 1991 Oct 1;51(19):5329–5338. [PubMed] [Google Scholar]
- Connell N. D., Rheinwald J. G. Regulation of the cytoskeleton in mesothelial cells: reversible loss of keratin and increase in vimentin during rapid growth in culture. Cell. 1983 Aug;34(1):245–253. doi: 10.1016/0092-8674(83)90155-1. [DOI] [PubMed] [Google Scholar]
- Czernobilsky B., Lifschitz-Mercer B., Luzon A., Jacob N., Ben-Hur H., Gorbacz S., Fogel M. Cytokeratin patterns in the epidermis of human ovarian mature cystic teratomas. Hum Pathol. 1989 Feb;20(2):185–192. doi: 10.1016/0046-8177(89)90184-6. [DOI] [PubMed] [Google Scholar]
- Czernobilsky B., Moll R., Levy R., Franke W. W. Co-expression of cytokeratin and vimentin filaments in mesothelial, granulosa and rete ovarii cells of the human ovary. Eur J Cell Biol. 1985 May;37:175–190. [PubMed] [Google Scholar]
- Di Bello M., Lucchini V., Chiari S., Colleoni R., Colombo N., Mantovani A., Allavena P. DR antigen expression on ovarian carcinoma cells does not correlate with their capacity to elicit an autologous proliferative response. Cancer Immunol Immunother. 1988;27(1):63–68. doi: 10.1007/BF00205760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrant L. G., Ballantyne K. C., Armitage N. C., Robins R. A., Marksman R., Hardcastle J. D., Baldwin R. W. Quantitation of MHC antigen expression on colorectal tumours and its association with tumour progression. Br J Cancer. 1987 Oct;56(4):425–432. doi: 10.1038/bjc.1987.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleuren G. J., Coerkamp E. G., Nap M., vd Broek L. J., Warnaar S. O. Immunohistological characterization of a monoclonal antibody (OV632) against epithelial ovarian carcinomas. Virchows Arch A Pathol Anat Histopathol. 1987;410(6):481–486. doi: 10.1007/BF00781682. [DOI] [PubMed] [Google Scholar]
- Heinemann D., Smith P. J., Symes M. O. Expression of histocompatibility antigens and characterisation of mononuclear cell infiltrates in human renal cell carcinomas. Br J Cancer. 1987 Oct;56(4):433–437. doi: 10.1038/bjc.1987.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämmerling G. J., Klar D., Pülm W., Momburg F., Moldenhauer G. The influence of major histocompatibility complex class I antigens on tumor growth and metastasis. Biochim Biophys Acta. 1987 Nov 25;907(3):245–259. doi: 10.1016/0304-419x(87)90008-4. [DOI] [PubMed] [Google Scholar]
- Ivanyi D., Ansink A., Groeneveld E., Hageman P. C., Mooi W. J., Heintz A. P. New monoclonal antibodies recognizing epidermal differentiation-associated keratins in formalin-fixed, paraffin-embedded tissue. Keratin 10 expression in carcinoma of the vulva. J Pathol. 1989 Sep;159(1):7–12. doi: 10.1002/path.1711590105. [DOI] [PubMed] [Google Scholar]
- Johnson V. G., Schlom J., Paterson A. J., Bennett J., Magnani J. L., Colcher D. Analysis of a human tumor-associated glycoprotein (TAG-72) identified by monoclonal antibody B72.3. Cancer Res. 1986 Feb;46(2):850–857. [PubMed] [Google Scholar]
- Johnston W. W., Szpak C. A., Lottich S. C., Thor A., Schlom J. Use of a monoclonal antibody (B72.3) as a novel immunohistochemical adjunct for the diagnosis of carcinomas in fine needle aspiration biopsy specimens. Hum Pathol. 1986 May;17(5):501–513. doi: 10.1016/s0046-8177(86)80041-7. [DOI] [PubMed] [Google Scholar]
- Kruitwagen R. F., Poels L. G., Willemsen W. N., Jap P. H., de Ronde I. J., Hanselaar T. G., Rolland R. Immunocytochemical markerprofile of endometriotic epithelial, endometrial epithelial, and mesothelial cells: a comparative study. Eur J Obstet Gynecol Reprod Biol. 1991 Oct 8;41(3):215–223. doi: 10.1016/0028-2243(91)90027-i. [DOI] [PubMed] [Google Scholar]
- Kruitwagen R. F., Poels L. G., Willemsen W. N., de Ronde I. J., Jap P. H., Rolland R. Endometrial epithelial cells in peritoneal fluid during the early follicular phase. Fertil Steril. 1991 Feb;55(2):297–303. doi: 10.1016/s0015-0282(16)54119-3. [DOI] [PubMed] [Google Scholar]
- LaRocca P. J., Rheinwald J. G. Coexpression of simple epithelial keratins and vimentin by human mesothelium and mesothelioma in vivo and in culture. Cancer Res. 1984 Jul;44(7):2991–2999. [PubMed] [Google Scholar]
- Miettinen M., Lehto V. P., Virtanen I. Expression of intermediate filaments in normal ovaries and ovarian epithelial, sex cord-stromal, and germinal tumors. Int J Gynecol Pathol. 1983;2(1):64–71. doi: 10.1097/00004347-198301000-00006. [DOI] [PubMed] [Google Scholar]
- Miller T. P., Lippman S. M., Spier C. M., Slymen D. J., Grogan T. M. HLA-DR (Ia) immune phenotype predicts outcome for patients with diffuse large cell lymphoma. J Clin Invest. 1988 Jul;82(1):370–372. doi: 10.1172/JCI113598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll R., Pitz S., Levy R., Weikel W., Franke W. W., Czernobilsky B. Complexity of expression of intermediate filament proteins, including glial filament protein, in endometrial and ovarian adenocarcinomas. Hum Pathol. 1991 Oct;22(10):989–1001. doi: 10.1016/0046-8177(91)90007-c. [DOI] [PubMed] [Google Scholar]
- Momburg F., Ziegler A., Harpprecht J., Möller P., Moldenhauer G., Hämmerling G. J. Selective loss of HLA-A or HLA-B antigen expression in colon carcinoma. J Immunol. 1989 Jan 1;142(1):352–358. [PubMed] [Google Scholar]
- Napolitano L. A., Vogel J., Jay G. The role of major histocompatibility complex class I antigens in tumorigenesis: future applications in cancer therapy. Biochim Biophys Acta. 1989 Dec 17;989(2):153–162. doi: 10.1016/0304-419x(89)90040-1. [DOI] [PubMed] [Google Scholar]
- Osborn M., Weber K. Tumor diagnosis by intermediate filament typing: a novel tool for surgical pathology. Lab Invest. 1983 Apr;48(4):372–394. [PubMed] [Google Scholar]
- Pieper F. R., Schaart G., Krimpenfort P. J., Henderik J. B., Moshage H. J., van de Kemp A., Ramaekers F. C., Berns A., Bloemendal H. Transgenic expression of the muscle-specific intermediate filament protein desmin in nonmuscle cells. J Cell Biol. 1989 Mar;108(3):1009–1024. doi: 10.1083/jcb.108.3.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poels L. G., Peters D., van Megen Y., Vooijs G. P., Verheyen R. N., Willemen A., van Niekerk C. C., Jap P. H., Mungyer G., Kenemans P. Monoclonal antibody against human ovarian tumor-associated antigens. J Natl Cancer Inst. 1986 May;76(5):781–791. [PubMed] [Google Scholar]
- Purkis P. E., Steel J. B., Mackenzie I. C., Nathrath W. B., Leigh I. M., Lane E. B. Antibody markers of basal cells in complex epithelia. J Cell Sci. 1990 Sep;97(Pt 1):39–50. doi: 10.1242/jcs.97.1.39. [DOI] [PubMed] [Google Scholar]
- Puts J. J., Moesker O., Aldeweireldt J., Vooijs G. P., Ramaekers F. C. Application of antibodies to intermediate filament proteins in simple and complex tumors of the female genital tract. Int J Gynecol Pathol. 1987;6(3):257–274. doi: 10.1097/00004347-198709000-00007. [DOI] [PubMed] [Google Scholar]
- Ramaekers F. C., Moesker O., Huysmans A., Schaart G., Westerhof G., Wagenaar S. S., Herman C. J., Vooijs G. P. Intermediate filament proteins in the study of tumor heterogeneity: an in-depth study of tumors of the urinary and respiratory tracts. Ann N Y Acad Sci. 1985;455:614–634. doi: 10.1111/j.1749-6632.1985.tb50440.x. [DOI] [PubMed] [Google Scholar]
- Ramaekers F. C., Puts J. J., Moesker O., Kant A., Huysmans A., Haag D., Jap P. H., Herman C. J., Vooijs G. P. Antibodies to intermediate filament proteins in the immunohistochemical identification of human tumours: an overview. Histochem J. 1983 Jul;15(7):691–713. doi: 10.1007/BF01002988. [DOI] [PubMed] [Google Scholar]
- Ramaekers F., Huysmans A., Moesker O., Kant A., Jap P., Herman C., Vooijs P. Monoclonal antibody to keratin filaments, specific for glandular epithelia and their tumors. Use in surgical pathology. Lab Invest. 1983 Sep;49(3):353–361. [PubMed] [Google Scholar]
- Ramaekers F., Huysmans A., Schaart G., Moesker O., Vooijs P. Tissue distribution of keratin 7 as monitored by a monoclonal antibody. Exp Cell Res. 1987 May;170(1):235–249. doi: 10.1016/0014-4827(87)90133-9. [DOI] [PubMed] [Google Scholar]
- Ramaekers F., Puts J., Moesker O., Kant A., Jap P., Vooijs P. Demonstration of keratin in human adenocarcinomas. Am J Pathol. 1983 May;111(2):213–223. [PMC free article] [PubMed] [Google Scholar]
- Ramaekers F., van Niekerk C., Poels L., Schaafsma E., Huijsmans A., Robben H., Schaart G., Vooijs P. Use of monoclonal antibodies to keratin 7 in the differential diagnosis of adenocarcinomas. Am J Pathol. 1990 Mar;136(3):641–655. [PMC free article] [PubMed] [Google Scholar]
- Ruiter D. J., Bröcker E. B., Ferrone S. Expression and susceptibility to modulation by interferons of HLA class I and II antigens on melanoma cells. Immunohistochemical analysis and clinical relevance. J Immunogenet. 1986 Apr-Jun;13(2-3):229–234. doi: 10.1111/j.1744-313x.1986.tb01105.x. [DOI] [PubMed] [Google Scholar]
- Schaafsma H. E., Ramaekers F. C., van Muijen G. N., Lane E. B., Leigh I. M., Robben H., Huijsmans A., Ooms E. C., Ruiter D. J. Distribution of cytokeratin polypeptides in human transitional cell carcinomas, with special emphasis on changing expression patterns during tumor progression. Am J Pathol. 1990 Feb;136(2):329–343. [PMC free article] [PubMed] [Google Scholar]
- Schaart G., Viebahn C., Langmann W., Ramaekers F. Desmin and titin expression in early postimplantation mouse embryos. Development. 1989 Nov;107(3):585–596. doi: 10.1242/dev.107.3.585. [DOI] [PubMed] [Google Scholar]
- Smedts F., Ramaekers F., Robben H., Pruszczynski M., van Muijen G., Lane B., Leigh I., Vooijs P. Changing patterns of keratin expression during progression of cervical intraepithelial neoplasia. Am J Pathol. 1990 Mar;136(3):657–668. [PMC free article] [PubMed] [Google Scholar]
- Stasiak P. C., Purkis P. E., Leigh I. M., Lane E. B. Keratin 19: predicted amino acid sequence and broad tissue distribution suggest it evolved from keratinocyte keratins. J Invest Dermatol. 1989 May;92(5):707–716. doi: 10.1111/1523-1747.ep12721500. [DOI] [PubMed] [Google Scholar]
- Szpak C. A., Johnston W. W., Roggli V., Kolbeck J., Lottich S. C., Vollmer R., Thor A., Schlom J. The diagnostic distinction between malignant mesothelioma of the pleura and adenocarcinoma of the lung as defined by a monoclonal antibody (B72.3). Am J Pathol. 1986 Feb;122(2):252–260. [PMC free article] [PubMed] [Google Scholar]
- Taylor-Papadimitriou J., Peterson J. A., Arklie J., Burchell J., Ceriani R. L., Bodmer W. F. Monoclonal antibodies to epithelium-specific components of the human milk fat globule membrane: production and reaction with cells in culture. Int J Cancer. 1981 Jul 15;28(1):17–21. doi: 10.1002/ijc.2910280104. [DOI] [PubMed] [Google Scholar]
- Valea F. A., Haskill S., Olafsson K., Fowler W. C., Jr Flow cytometric versus immunohistochemical analysis of ovarian cancer class I antigen expression: differences may represent a defect in antigen expression. Gynecol Oncol. 1990 Sep;38(3):413–420. doi: 10.1016/0090-8258(90)90083-w. [DOI] [PubMed] [Google Scholar]
- Van Muijen G. N., Ruiter D. J., Warnaar S. O. Coexpression of intermediate filament polypeptides in human fetal and adult tissues. Lab Invest. 1987 Oct;57(4):359–369. [PubMed] [Google Scholar]
- Viale G., Gambacorta M., Dell'Orto P., Coggi G. Coexpression of cytokeratins and vimentin in common epithelial tumours of the ovary: an immunocytochemical study of eighty-three cases. Virchows Arch A Pathol Anat Histopathol. 1988;413(2):91–101. doi: 10.1007/BF00749670. [DOI] [PubMed] [Google Scholar]
- Vánky F., Klein E., Willems J. DR antigens expressed on tumor cells do not contribute to the blastogenetic response of autologous T cells. Cancer Immunol Immunother. 1985;19(3):219–225. doi: 10.1007/BF00199230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzels R. H., Kuijpers H. J., Lane E. B., Leigh I. M., Troyanovsky S. M., Holland R., van Haelst U. J., Ramaekers F. C. Basal cell-specific and hyperproliferation-related keratins in human breast cancer. Am J Pathol. 1991 Mar;138(3):751–763. [PMC free article] [PubMed] [Google Scholar]
- van Muijen G. N., Ruiter D. J., Franke W. W., Achtstätter T., Haasnoot W. H., Ponec M., Warnaar S. O. Cell type heterogeneity of cytokeratin expression in complex epithelia and carcinomas as demonstrated by monoclonal antibodies specific for cytokeratins nos. 4 and 13. Exp Cell Res. 1986 Jan;162(1):97–113. doi: 10.1016/0014-4827(86)90429-5. [DOI] [PubMed] [Google Scholar]
- van Muijen G. N., Ruiter D. J., van Leeuwen C., Prins F. A., Rietsema K., Warnaar S. O. Cytokeratin and neurofilament in lung carcinomas. Am J Pathol. 1984 Sep;116(3):363–369. [PMC free article] [PubMed] [Google Scholar]
- van Niekerk C. C., Boerman O. C., Ramaekers F. C., Poels L. G. Marker profile of different phases in the transition of normal human ovarian epithelium to ovarian carcinomas. Am J Pathol. 1991 Feb;138(2):455–463. [PMC free article] [PubMed] [Google Scholar]
- van Niekerk C. C., Jap P. H., Ramaekers F. C., van de Molengraft F., Poels L. G. Immunohistochemical demonstration of keratin 7 in routinely fixed paraffin-embedded human tissues. J Pathol. 1991 Oct;165(2):145–152. doi: 10.1002/path.1711650210. [DOI] [PubMed] [Google Scholar]
- van Niekerk C. C., Jap P. H., Thomas C. M., Smeets D. F., Ramaekers F. C., Poels L. G. Marker profile of mesothelial cells versus ovarian carcinoma cells. Int J Cancer. 1989 Jun 15;43(6):1065–1071. doi: 10.1002/ijc.2910430619. [DOI] [PubMed] [Google Scholar]
- van de Molengraft F., Ramaekers F., Jap P., Vooijs P., Mungyer G. Changing intermediate-sized filament patterns in metastatic hepatocellular carcinoma cells of the guinea pig. Virchows Arch B Cell Pathol Incl Mol Pathol. 1986;51(4):285–301. doi: 10.1007/BF02899038. [DOI] [PubMed] [Google Scholar]