Abstract
The transforming growth factor (TGF)-betas are a highly conserved group of potent multifunctional cell regulatory proteins with variable effects on cell growth and differentiation. Most of the small round cell group of childhood tumors are thought to arise from either primitive mesenchyme or neuroectoderm and show evidence of skeletal muscle or neural differentiation, and rarely both. To investigate the possibility that the TGF-betas have a role in the growth or differentiation of these neoplasms, we used antibodies specific for peptide sequences of the three known mammalian TGF-beta isoforms (TGF-betas 1, 2, and 3) to probe for TGF-beta protein expression in a total of 49 cases. TGF-beta 1 immunoreactivity was present in 16/17 (94%) of rhabdomyosarcomas, and the staining intensity was usually strong. TGF-beta 1 was also present in three of three ectomesenchymomas. In contrast, TGF-beta 1 was absent in all but one out of nine poorly differentiated neuroblastomas. Differentiating neuronal cells of ganglioneuroblastomas, however, were strongly positive for TGF-beta 1. Ewing's sarcomas and peripheral primitive neuroectodermal tumors had a less consistent, but usually positive, staining pattern. TGF-beta 3 staining patterns were very similar to those of TGF-beta 1. TGF-beta 2 immunoreactivity was only rarely detected in this group of tumors. The results suggest different roles for TGF-betas 1 and 3 in neuroblastoma and rhabdomyosarcoma. Expression of TGF-betas 1 and 3 is associated with neuronal differentiation of neuroblastoma. In contrast, these proteins may promote the growth of rhabdomyosarcoma by suppressing differentiation.
Full text
PDF

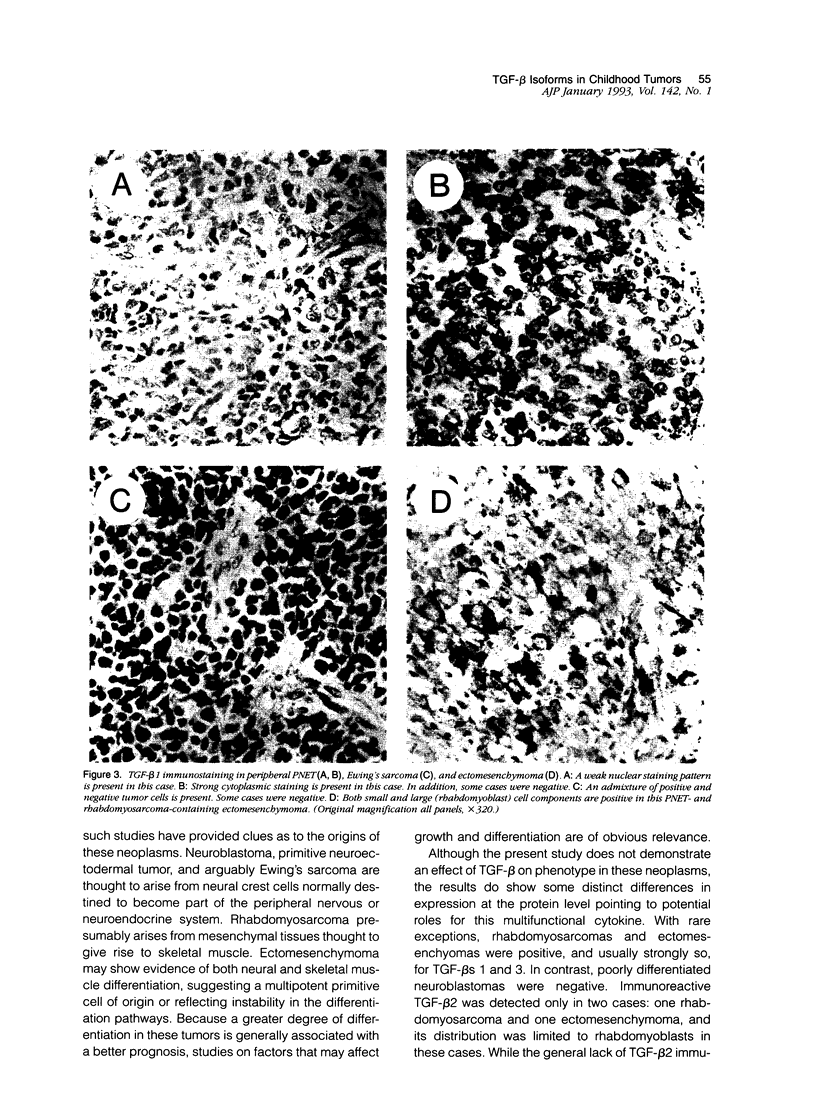



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. E., Boxhorn L. K. Regulation of skeletal muscle satellite cell proliferation and differentiation by transforming growth factor-beta, insulin-like growth factor I, and fibroblast growth factor. J Cell Physiol. 1989 Feb;138(2):311–315. doi: 10.1002/jcp.1041380213. [DOI] [PubMed] [Google Scholar]
- Assoian R. K., Komoriya A., Meyers C. A., Miller D. M., Sporn M. B. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem. 1983 Jun 10;258(11):7155–7160. [PubMed] [Google Scholar]
- Baker D. L., Reddy U. R., Pleasure D., Thorpe C. L., Evans A. E., Cohen P. S., Ross A. H. Analysis of nerve growth factor receptor expression in human neuroblastoma and neuroepithelioma cell lines. Cancer Res. 1989 Aug 1;49(15):4142–4146. [PubMed] [Google Scholar]
- Cattaneo E., McKay R. Proliferation and differentiation of neuronal stem cells regulated by nerve growth factor. Nature. 1990 Oct 25;347(6295):762–765. doi: 10.1038/347762a0. [DOI] [PubMed] [Google Scholar]
- Cheifetz S., Weatherbee J. A., Tsang M. L., Anderson J. K., Mole J. E., Lucas R., Massagué J. The transforming growth factor-beta system, a complex pattern of cross-reactive ligands and receptors. Cell. 1987 Feb 13;48(3):409–415. doi: 10.1016/0092-8674(87)90192-9. [DOI] [PubMed] [Google Scholar]
- Christiansen H., Christiansen N. M., Wagner F., Altmannsberger M., Lampert F. Neuroblastoma: inverse relationship between expression of N-myc and NGF-r. Oncogene. 1990 Mar;5(3):437–440. [PubMed] [Google Scholar]
- Davies A. M. Role of neurotrophic factors in development. Trends Genet. 1988 May;4(5):139–143. doi: 10.1016/0168-9525(88)90137-0. [DOI] [PubMed] [Google Scholar]
- Derynck R., Lindquist P. B., Lee A., Wen D., Tamm J., Graycar J. L., Rhee L., Mason A. J., Miller D. A., Coffey R. J. A new type of transforming growth factor-beta, TGF-beta 3. EMBO J. 1988 Dec 1;7(12):3737–3743. doi: 10.1002/j.1460-2075.1988.tb03257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias P., Parham D. M., Shapiro D. N., Webber B. L., Houghton P. J. Myogenic regulatory protein (MyoD1) expression in childhood solid tumors: diagnostic utility in rhabdomyosarcoma. Am J Pathol. 1990 Dec;137(6):1283–1291. [PMC free article] [PubMed] [Google Scholar]
- El-Badry O. M., Romanus J. A., Helman L. J., Cooper M. J., Rechler M. M., Israel M. A. Autonomous growth of a human neuroblastoma cell line is mediated by insulin-like growth factor II. J Clin Invest. 1989 Sep;84(3):829–839. doi: 10.1172/JCI114243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fafeur V., Terman B. I., Blum J., Böhlen P. Basic FGF treatment of endothelial cells down-regulates the 85-KDa TGF beta receptor subtype and decreases the growth inhibitory response to TGF-beta 1. Growth Factors. 1990;3(3):237–245. doi: 10.3109/08977199009043908. [DOI] [PubMed] [Google Scholar]
- Flanders K. C., Cissel D. S., Mullen L. T., Danielpour D., Sporn M. B., Roberts A. B. Antibodies to transforming growth factor-beta 2 peptides: specific detection of TGF-beta 2 in immunoassays. Growth Factors. 1990;3(1):45–52. doi: 10.3109/08977199009037501. [DOI] [PubMed] [Google Scholar]
- Flanders K. C., Lüdecke G., Engels S., Cissel D. S., Roberts A. B., Kondaiah P., Lafyatis R., Sporn M. B., Unsicker K. Localization and actions of transforming growth factor-beta s in the embryonic nervous system. Development. 1991 Sep;113(1):183–191. doi: 10.1242/dev.113.1.183. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Plouët J., Malerstein B. Comparison of the ability of basic and acidic fibroblast growth factor to stimulate the proliferation of an established keratinocyte cell line: modulation of their biological effects by heparin, transforming growth factor beta (TGF beta), and epidermal growth factor (EGF). J Cell Physiol. 1990 Feb;142(2):325–333. doi: 10.1002/jcp.1041420215. [DOI] [PubMed] [Google Scholar]
- Heine U., Munoz E. F., Flanders K. C., Ellingsworth L. R., Lam H. Y., Thompson N. L., Roberts A. B., Sporn M. B. Role of transforming growth factor-beta in the development of the mouse embryo. J Cell Biol. 1987 Dec;105(6 Pt 2):2861–2876. doi: 10.1083/jcb.105.6.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heino J., Massagué J. Cell adhesion to collagen and decreased myogenic gene expression implicated in the control of myogenesis by transforming growth factor beta. J Biol Chem. 1990 Jun 25;265(18):10181–10184. [PubMed] [Google Scholar]
- Horton W. E., Jr, Higginbotham J. D., Chandrasekhar S. Transforming growth factor-beta and fibroblast growth factor act synergistically to inhibit collagen II synthesis through a mechanism involving regulatory DNA sequences. J Cell Physiol. 1989 Oct;141(1):8–15. doi: 10.1002/jcp.1041410103. [DOI] [PubMed] [Google Scholar]
- Kim S. J., Glick A., Sporn M. B., Roberts A. B. Characterization of the promoter region of the human transforming growth factor-beta 1 gene. J Biol Chem. 1989 Jan 5;264(1):402–408. [PubMed] [Google Scholar]
- Lafyatis R., Lechleider R., Kim S. J., Jakowlew S., Roberts A. B., Sporn M. B. Structural and functional characterization of the transforming growth factor beta 3 promoter. A cAMP-responsive element regulates basal and induced transcription. J Biol Chem. 1990 Nov 5;265(31):19128–19136. [PubMed] [Google Scholar]
- Lehnert S. A., Akhurst R. J. Embryonic expression pattern of TGF beta type-1 RNA suggests both paracrine and autocrine mechanisms of action. Development. 1988 Oct;104(2):263–273. doi: 10.1242/dev.104.2.263. [DOI] [PubMed] [Google Scholar]
- Malipiero U., Höller M., Werner U., Fontana A. Sequence analysis of the promoter region of the glioblastoma derived T cell suppressor factor/transforming growth factor (TGF)-beta 2 gene reveals striking differences to the TGF-beta 1 and -beta 3 genes. Biochem Biophys Res Commun. 1990 Sep 28;171(3):1145–1151. doi: 10.1016/0006-291x(90)90804-v. [DOI] [PubMed] [Google Scholar]
- Massagué J., Cheifetz S., Endo T., Nadal-Ginard B. Type beta transforming growth factor is an inhibitor of myogenic differentiation. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8206–8210. doi: 10.1073/pnas.83.21.8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- Matsushima H., Bogenmann E. Nerve growth factor (NGF) induces neuronal differentiation in neuroblastoma cells transfected with the NGF receptor cDNA. Mol Cell Biol. 1990 Sep;10(9):5015–5020. doi: 10.1128/mcb.10.9.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune B. K., Mullin B. R., Flanders K. C., Jaffurs W. J., Mullen L. T., Sporn M. B. Localization of transforming growth factor-beta isotypes in lesions of the human breast. Hum Pathol. 1992 Jan;23(1):13–20. doi: 10.1016/0046-8177(92)90004-m. [DOI] [PubMed] [Google Scholar]
- Millan F. A., Denhez F., Kondaiah P., Akhurst R. J. Embryonic gene expression patterns of TGF beta 1, beta 2 and beta 3 suggest different developmental functions in vivo. Development. 1991 Jan;111(1):131–143. doi: 10.1242/dev.111.1.131. [DOI] [PubMed] [Google Scholar]
- Moore J. W., Dionne C., Jaye M., Swain J. L. The mRNAs encoding acidic FGF, basic FGF and FGF receptor are coordinately downregulated during myogenic differentiation. Development. 1991 Mar;111(3):741–748. doi: 10.1242/dev.111.3.741. [DOI] [PubMed] [Google Scholar]
- Olson E. N., Sternberg E., Hu J. S., Spizz G., Wilcox C. Regulation of myogenic differentiation by type beta transforming growth factor. J Cell Biol. 1986 Nov;103(5):1799–1805. doi: 10.1083/jcb.103.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper M. S., Belin D., Montesano R., Orci L., Vassalli J. D. Transforming growth factor-beta 1 modulates basic fibroblast growth factor-induced proteolytic and angiogenic properties of endothelial cells in vitro. J Cell Biol. 1990 Aug;111(2):743–755. doi: 10.1083/jcb.111.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plouët J., Gospodarowicz D. Transforming growth factor beta-1 positively modulates the bioactivity of fibroblast growth factor on corneal endothelial cells. J Cell Physiol. 1989 Nov;141(2):392–399. doi: 10.1002/jcp.1041410221. [DOI] [PubMed] [Google Scholar]
- Påhlman S., Odelstad L., Larsson E., Grotte G., Nilsson K. Phenotypic changes of human neuroblastoma cells in culture induced by 12-O-tetradecanoyl-phorbol-13-acetate. Int J Cancer. 1981 Nov 15;28(5):583–589. doi: 10.1002/ijc.2910280509. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Wakefield L. M., Roche N. S., Stern D. F., Sporn M. B. Type beta transforming growth factor: a bifunctional regulator of cellular growth. Proc Natl Acad Sci U S A. 1985 Jan;82(1):119–123. doi: 10.1073/pnas.82.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid P., Cox D., Bilbe G., Maier R., McMaster G. K. Differential expression of TGF beta 1, beta 2 and beta 3 genes during mouse embryogenesis. Development. 1991 Jan;111(1):117–130. doi: 10.1242/dev.111.1.117. [DOI] [PubMed] [Google Scholar]
- Schweigerer L., Neufeld G., Mergia A., Abraham J. A., Fiddes J. C., Gospodarowicz D. Basic fibroblast growth factor in human rhabdomyosarcoma cells: implications for the proliferation and neovascularization of myoblast-derived tumors. Proc Natl Acad Sci U S A. 1987 Feb;84(3):842–846. doi: 10.1073/pnas.84.3.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenfeld K. H., Ishii D. N. Nerve growth factor effects and receptors in cultured human neuroblastoma cell lines. J Neurosci Res. 1982;8(2-3):375–391. doi: 10.1002/jnr.490080226. [DOI] [PubMed] [Google Scholar]
- Tollefsen S. E., Lajara R., McCusker R. H., Clemmons D. R., Rotwein P. Insulin-like growth factors (IGF) in muscle development. Expression of IGF-I, the IGF-I receptor, and an IGF binding protein during myoblast differentiation. J Biol Chem. 1989 Aug 15;264(23):13810–13817. [PubMed] [Google Scholar]
- Tsokos M. Peripheral primitive neuroectodermal tumors. Diagnosis, classification, and prognosis. Perspect Pediatr Pathol. 1992;16:27–98. [PubMed] [Google Scholar]
- Tsokos M., Scarpa S., Ross R. A., Triche T. J. Differentiation of human neuroblastoma recapitulates neural crest development. Study of morphology, neurotransmitter enzymes, and extracellular matrix proteins. Am J Pathol. 1987 Sep;128(3):484–496. [PMC free article] [PubMed] [Google Scholar]
- Vaidya T. B., Rhodes S. J., Taparowsky E. J., Konieczny S. F. Fibroblast growth factor and transforming growth factor beta repress transcription of the myogenic regulatory gene MyoD1. Mol Cell Biol. 1989 Aug;9(8):3576–3579. doi: 10.1128/mcb.9.8.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield L. M., Smith D. M., Masui T., Harris C. C., Sporn M. B. Distribution and modulation of the cellular receptor for transforming growth factor-beta. J Cell Biol. 1987 Aug;105(2):965–975. doi: 10.1083/jcb.105.2.965. [DOI] [PMC free article] [PubMed] [Google Scholar]





