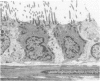Abstract
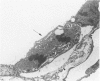
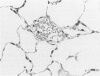
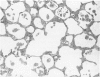
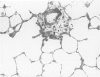
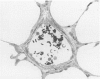
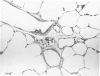
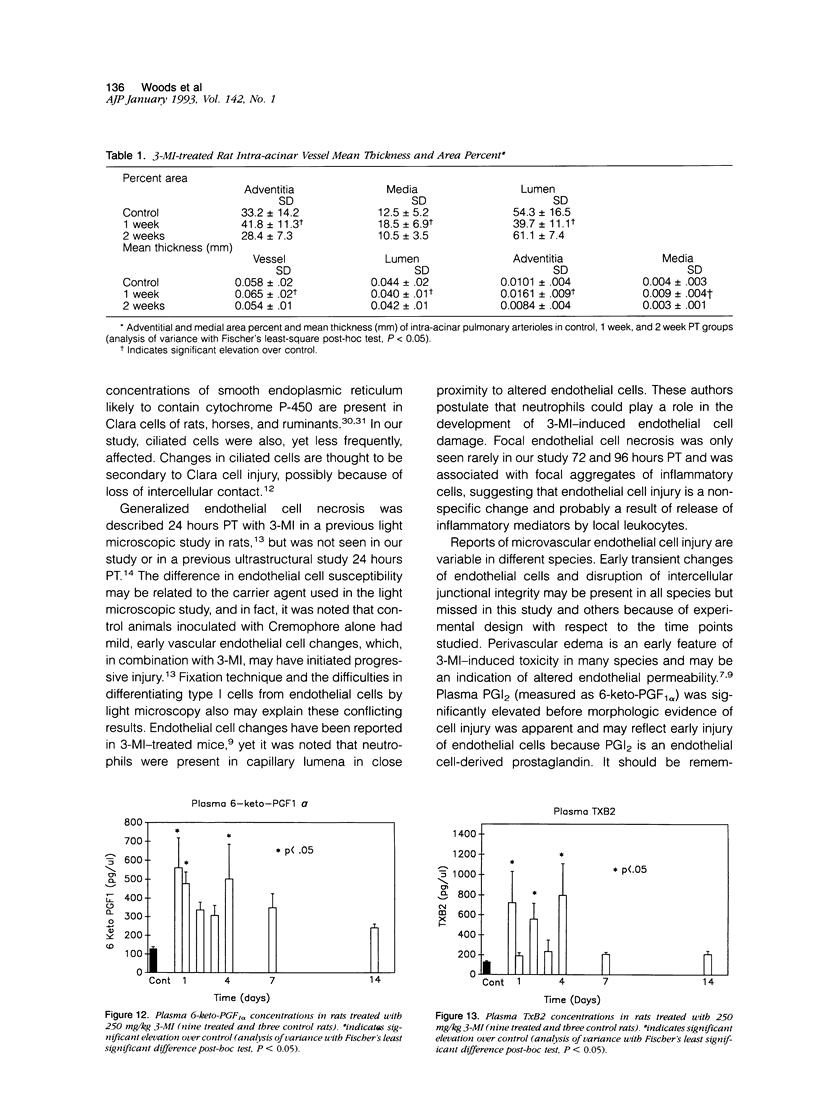
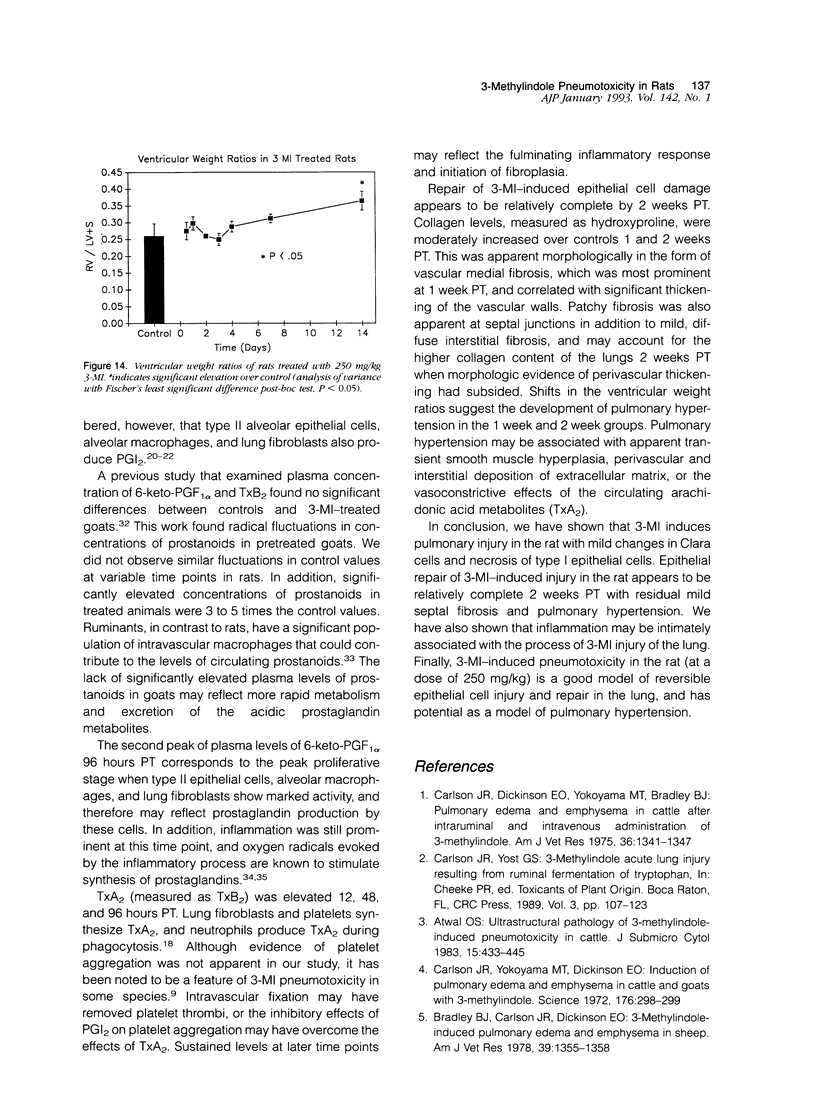
Effects of a single dose of 3-methylindole (3-MI) (250 mg/kg intraperitoneally) were studied at different times ranging from 12 hours to 2 weeks post-treatment (PT). Microscopic study revealed mild Clara cell injury 24 hours PT and mucus hyperplasia 24 hours to 2 weeks PT. Diffuse type I alveolar epithelial cell necrosis occurred at 48 hours, followed by type II cell hyperplasia. Septal edema and accumulation of interstitial and capillary polymorphonuclear leukocytes and perivascular mixed mononuclear inflammatory cells accompanied the injury and repair. A gradual resolution of lesions with persistent mononuclear inflammatory cellular clusters at septal junctions, focal septal fibrosis, and accumulation of alveolar macrophages was evident at 1 and 2 weeks PT. Collagen, measured as hydroxyproline, in 3-MI-treated rats was significantly increased to 130% and 139% of control (3.0 mg/lung) at 1 and 2 weeks PT, respectively. Biphasic peaks of plasma 6-keto-prostaglandin F1 alpha occurred at 12 to 24 hours and at 96 hours PT with 3-MI and thromboxane B2 was elevated 12, 48, and 96 hours PT. Right ventricular/left ventricular and septal weight was increased to 120% and 140% of the control 1 and 2 weeks PT. We concluded that 3-MI induces alveolar septal injury in the rat with relatively complete repair of the alveolar epithelium and residual mild focal septal fibrosis and pulmonary hypertension 2 weeks PT. Arachidonic acid-derived mediators and inflammation are associated with 3-MI-induced lung injury.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acton K. S., Bray T. M., Boermans H. J. Effect of 3-methylindole on the plasma and lung concentrations of prostaglandins and thromboxane B2 in goats. Comp Biochem Physiol A Comp Physiol. 1989;94(4):677–681. doi: 10.1016/0300-9629(89)90616-6. [DOI] [PubMed] [Google Scholar]
- Adams J. D., Jr, Laegreid W. W., Huijzer J. C., Hayman C., Yost G. S. Pathology and glutathione status in 3-methylindole-treated rodents. Res Commun Chem Pathol Pharmacol. 1988 Jun;60(3):323–336. [PubMed] [Google Scholar]
- Atwal O. S., Persofsky M. S. Ultrastructural changes in intraacinar pulmonary veins. Relationship to 3-methylindole-induced acute pulmonary edema and pulmonary arterial changes in cattle. Am J Pathol. 1984 Mar;114(3):472–486. [PMC free article] [PubMed] [Google Scholar]
- Atwal O. S., Persofsky M. S. Ultrastructural pathology of intrapulmonary arteries in 3-methylindole-induced pneumotoxicity in cattle: II. Glycogen accumulation in the smooth muscle cells and intimal changes. J Pathol. 1984 Feb;142(2):141–149. doi: 10.1002/path.1711420205. [DOI] [PubMed] [Google Scholar]
- Atwal O. S. Ultrastructural pathology of 3-methylindole-induced pneumotoxicity in cattle. I. Glycogen accumulation in the alveolar type II cells and tubular myelin accumulation in the alveoli. J Submicrosc Cytol. 1983 Apr;15(2):433–445. [PubMed] [Google Scholar]
- Bradley B. J., Carlson J. R., Dickinson E. O. 3-methylindole-induced pulmonary edema and emphysema in sheep. Am J Vet Res. 1978 Aug;39(8):1355–1358. [PubMed] [Google Scholar]
- Bradley B. J., Carlson J. R. Ultrastructural pulmonary changes induced by intravenously administered 3-methylindole in goats. Am J Pathol. 1980 Jun;99(3):551–560. [PMC free article] [PubMed] [Google Scholar]
- Carlson J. R., Dickinson E. O., Yokoyama M. T., Bradley B. Pulmonary edema and emphysema in cattle after intraruminal and intravenous administration of 3-methylindole. Am J Vet Res. 1975 Sep;36(9):1341–1347. [PubMed] [Google Scholar]
- Carlson J. R., Yokoyama M. T., Dickinson E. O. Induction of pulmonary edema and emphysema in cattle and goats with 3-methylindole. Science. 1972 Apr 21;176(4032):298–299. doi: 10.1126/science.176.4032.298. [DOI] [PubMed] [Google Scholar]
- Chandler D. B., Giri S. N. Changes in plasma concentrations of prostaglandins and plasma angiotensin-converting enzyme during bleomycin-induced lung fibrosis in hamsters. Am Rev Respir Dis. 1983 Jul;128(1):71–76. doi: 10.1164/arrd.1983.128.1.71. [DOI] [PubMed] [Google Scholar]
- Deby-Dupont G., Radoux L., Haas M., Larbuisson R., Noël F. X., Lamy M. Release of thromboxane B2 during adult respiratory distress syndrome and its inhibition by non steroidal anti-inflammatory substances in man. Arch Int Pharmacodyn Ther. 1982 Oct;259(2):317–319. [PubMed] [Google Scholar]
- Durham S. K., Castleman W. L. Pulmonary lesions induced by 3-methylindole in mice. Am J Pathol. 1985 Oct;121(1):128–137. [PMC free article] [PubMed] [Google Scholar]
- Hopkins N. K., Sun F. F., Gorman R. R. Thromboxane A2 biosynthesis in human lung fibroblasts WI-38. Biochem Biophys Res Commun. 1978 Nov 29;85(2):827–836. doi: 10.1016/0006-291x(78)91237-8. [DOI] [PubMed] [Google Scholar]
- Hsueh W. Prostaglandin biosynthesis in pulmonary macrophages. Am J Pathol. 1979 Oct;97(1):137–148. [PMC free article] [PubMed] [Google Scholar]
- Huang T. W., Carlson J. R., Bray T. M., Bradley B. J. 3-methylindole-induced pulmonary injury in goats. Am J Pathol. 1977 Jun;87(3):647–666. [PMC free article] [PubMed] [Google Scholar]
- Jones K. G., Holland J. F., Foureman G. L., Bend J. R., Fouts J. R. Xenobiotic metabolism in Clara cells and alveolar type II cells isolated from lungs of rats treated with beta-naphthoflavone. J Pharmacol Exp Ther. 1983 May;225(2):316–319. [PubMed] [Google Scholar]
- Kiorpes A. L., Keith I. M., Dubielzig R. R. Pulmonary changes in rats following administration of 3-methylindole in cremophore EL. Histol Histopathol. 1988 Apr;3(2):125–132. [PubMed] [Google Scholar]
- Lemen R. J., Gates A. J., Mathé A. A., Waring W. W., Hyman A. L., Kadowitz P. D. Relationships among digital clubbing, disease severity, and serum prostaglandins F2alpha and E concentrations in cystic fibrosis patients. Am Rev Respir Dis. 1978 Apr;117(4):639–646. doi: 10.1164/arrd.1978.117.4.639. [DOI] [PubMed] [Google Scholar]
- Nocerini M. R., Carlson J. R., Breeze R. G. Effect of glutathione status on covalent binding and pneumotoxicity of 3-methylindole in goats. Life Sci. 1983 Jan 31;32(5):449–458. doi: 10.1016/0024-3205(83)90137-6. [DOI] [PubMed] [Google Scholar]
- Panganamala R. V., Brownlee N. R., Sprecher H., Cornwell D. G. Evaluation of superoxide anion and singlet oxygen in the biosynthesis of prostaglandins from eicosa-8,11,14-trienoic acid. Prostaglandins. 1974 Jul 10;7(1):21–28. doi: 10.1016/s0090-6980(74)80074-2. [DOI] [PubMed] [Google Scholar]
- Rahimtula A., O'Brien P. J. The possible involvement of singlet oxygen in prostaglandin biosynthesis. Biochem Biophys Res Commun. 1976 Jun 7;70(3):893–899. doi: 10.1016/0006-291x(76)90675-6. [DOI] [PubMed] [Google Scholar]
- Slauson D. O. The mediation of pulmonary inflammatory injury. Adv Vet Sci Comp Med. 1982;26:99–153. [PubMed] [Google Scholar]
- Splawinski J., Gryglewski R. J. Release of prostacyclin by the lung. Bull Eur Physiopathol Respir. 1981;17(4):553–569. [PubMed] [Google Scholar]
- Taylor L., Polgar P., McAteer J. A., Douglas W. H. Prostaglandin production by type II alveolar epithelial cells. Biochim Biophys Acta. 1979 Mar 29;572(3):502–509. doi: 10.1016/0005-2760(79)90157-7. [DOI] [PubMed] [Google Scholar]
- Turk M. A., Breeze R. G., Gallina A. M. Pathologic changes in 3-methylindole-induced equine bronchiolitis. Am J Pathol. 1983 Feb;110(2):209–218. [PMC free article] [PubMed] [Google Scholar]
- Turk M. A., Flory W., Henk W. G. Dose response in 3-methylindole-induced bronchiolar epithelial necrosis in mice. Res Commun Chem Pathol Pharmacol. 1984 Dec;46(3):351–362. [PubMed] [Google Scholar]
- WOESSNER J. F., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961 May;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- Warner A. E., Barry B. E., Brain J. D. Pulmonary intravascular macrophages in sheep. Morphology and function of a novel constituent of the mononuclear phagocyte system. Lab Invest. 1986 Sep;55(3):276–288. [PubMed] [Google Scholar]
- Wilson D. W., Segall H. J., Pan L. C., Dunston S. K. Progressive inflammatory and structural changes in the pulmonary vasculature of monocrotaline-treated rats. Microvasc Res. 1989 Jul;38(1):57–80. doi: 10.1016/0026-2862(89)90017-4. [DOI] [PubMed] [Google Scholar]