Abstract
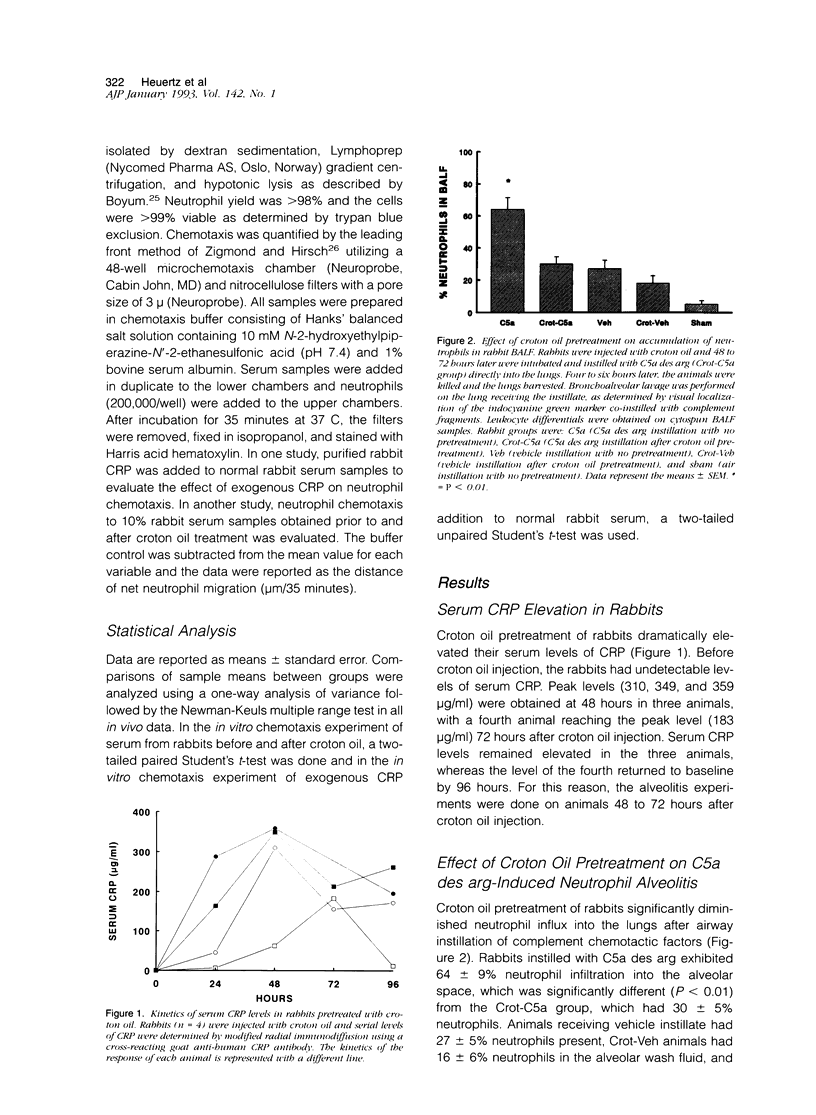
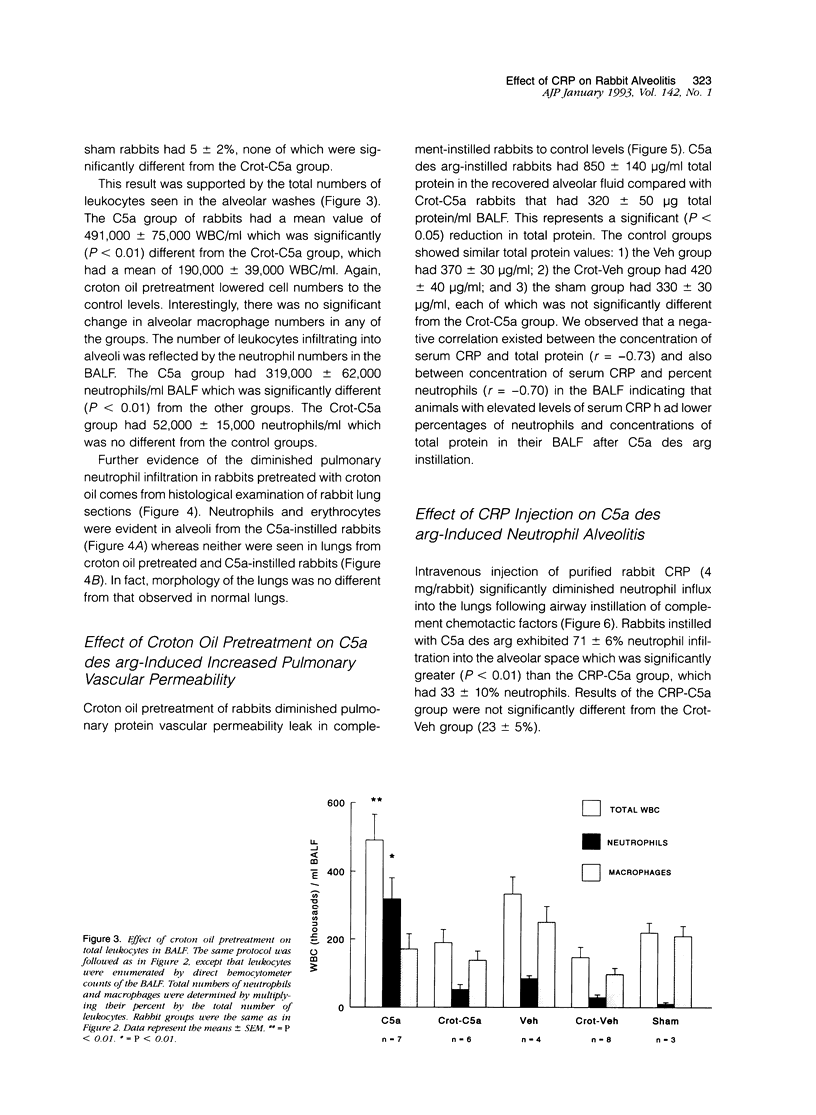
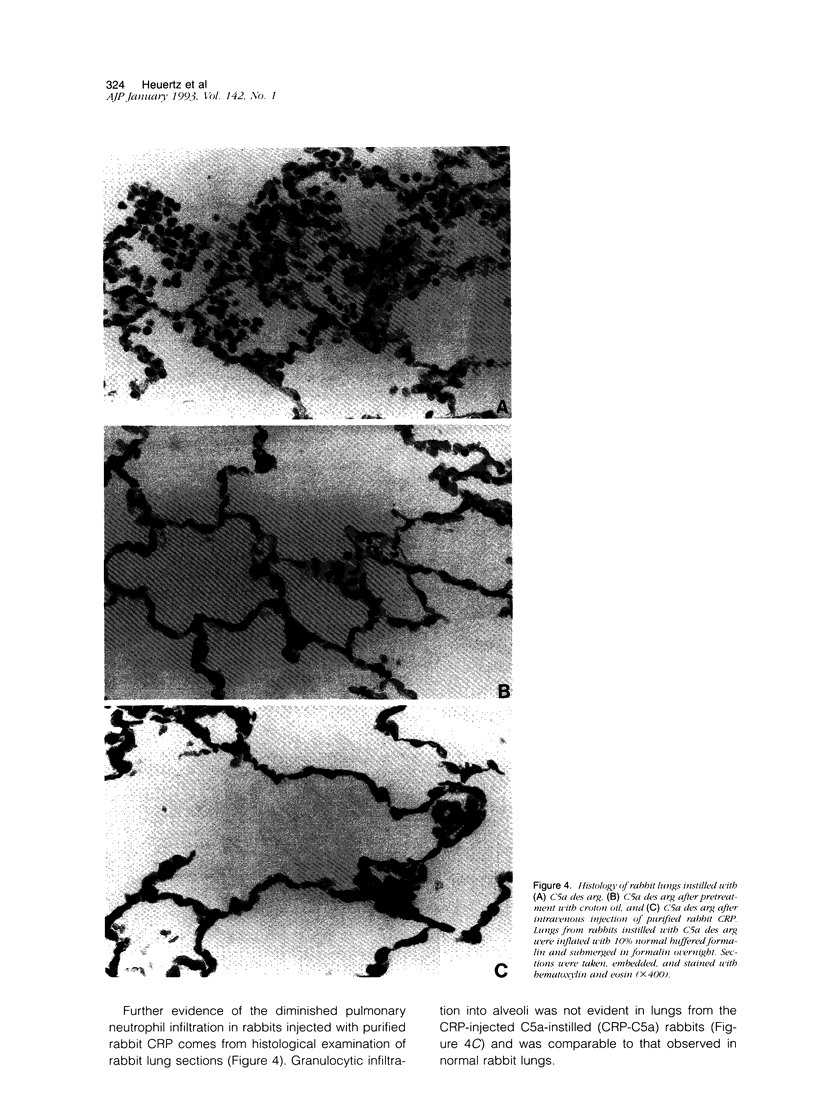
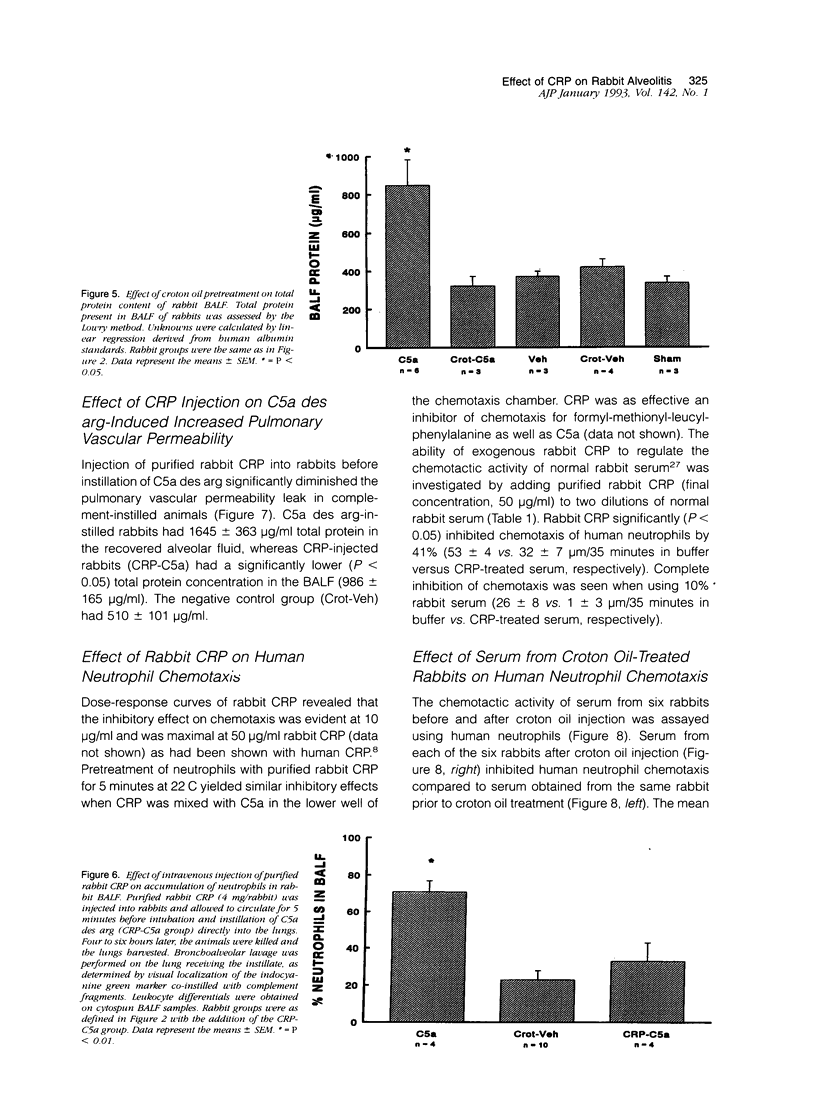
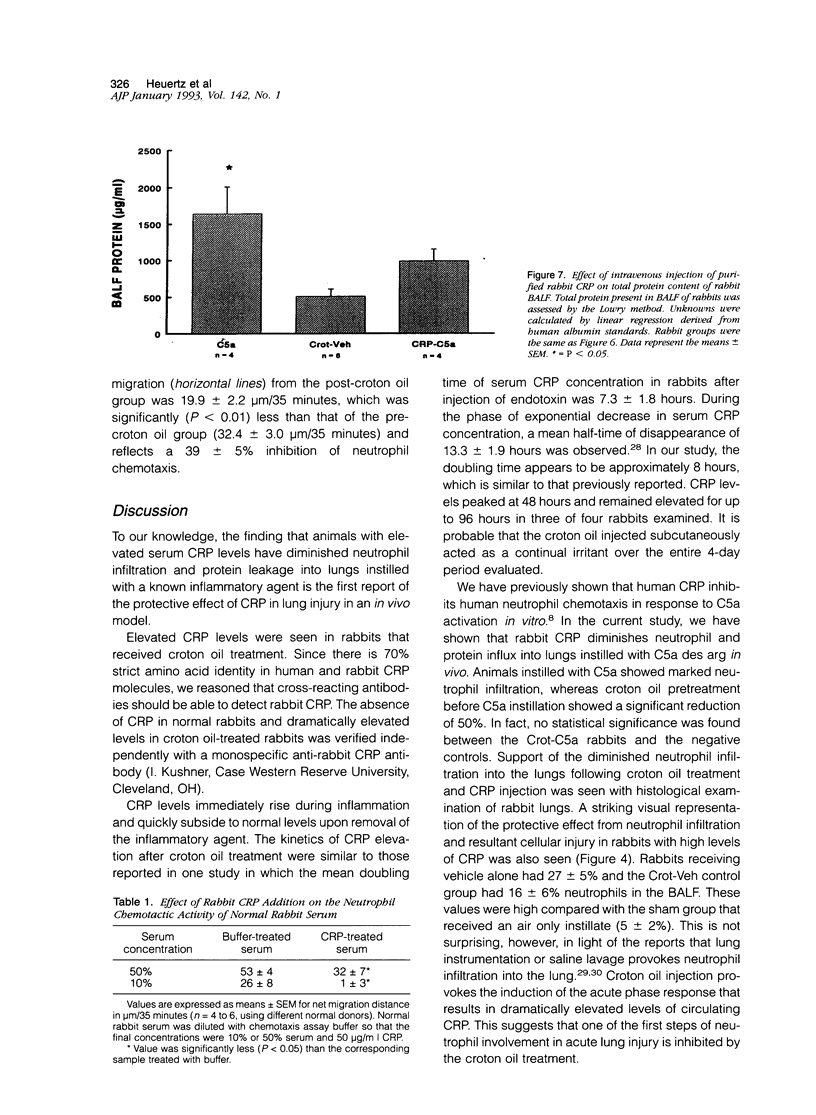
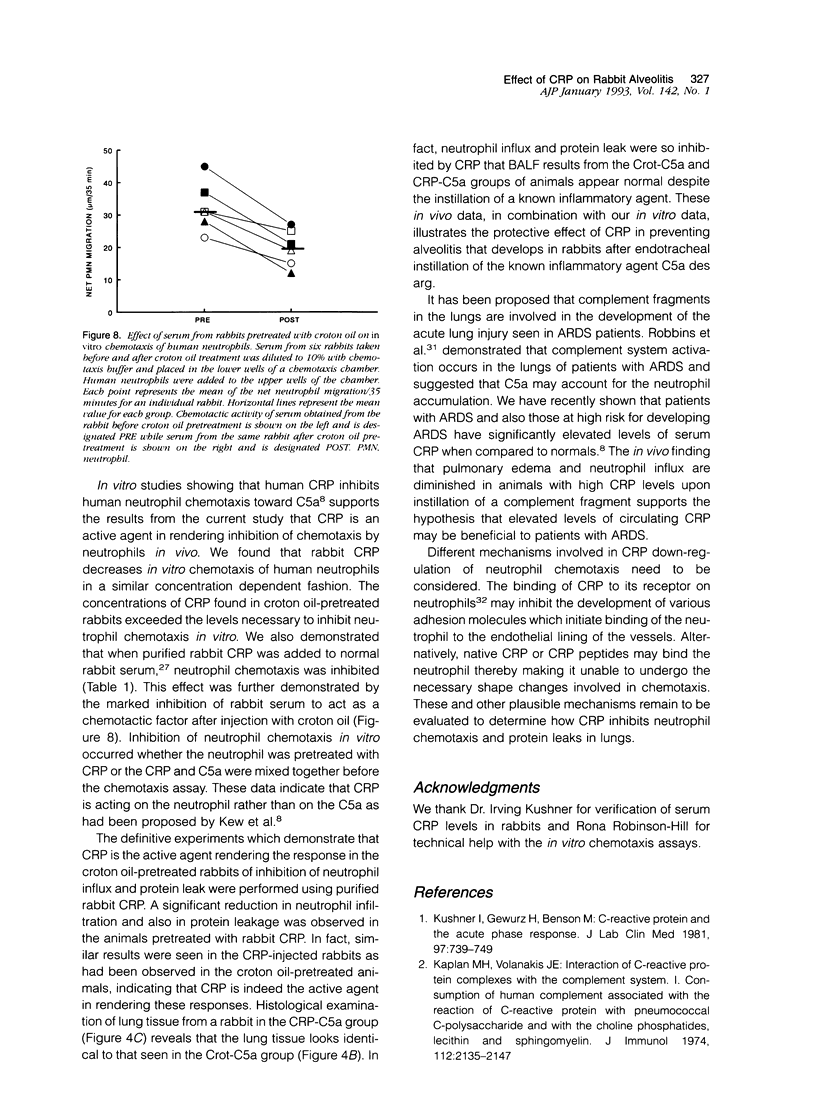
In previous studies, we have shown that C-reactive protein (CRP) inhibits chemotaxis of neutrophils to complement fragments in vitro. To evaluate the effect of CRP on C5a-induced inflammation in vivo, a rabbit model of alveolitis was used. Rabbits pretreated with subcutaneous injections of croton oil had serum CRP increase from undetectable levels to 270 +/- 70 micrograms/ml 48 hours later. Rabbits were intubated and C5a des arg (10 micrograms/ml) instilled directly into the lungs via an endotracheal tube. Four to six hours later, the animals were killed and bronchoalveolar lavage performed. Rabbits pretreated with croton oil had significantly (P < 0.01) reduced C5a des arg-stimulated neutrophil infiltration (30 +/- 5%) into alveolar air spaces compared to untreated rabbits (64 +/- 9%). Increased numbers of total leukocytes in the alveolar washes coincided with increased neutrophil numbers whereas alveolar macrophages remained unchanged in all groups. Rabbits pretreated with croton oil also had a significant decrease (P < 0.05) in total protein (320 +/- 50 micrograms/ml) in lavage fluid after C5a instillation compared with untreated animals (850 +/- 140 micrograms/ml). In vitro, rabbit CRP (50 micrograms/ml) added to normal rabbit serum significantly (P < 0.05) inhibited chemotaxis of human neutrophils by 41%. Finally, direct intravenous pretreatment of rabbits with purified CRP also significantly reduced C5a-induced alveolitis. The CRP-C5a group had 33 +/- 10% neutrophil infiltration, a significant (P < 0.01) reduction from the C5a group (71 +/- 6%). The total protein content of the CRP-C5a rabbits was 986 +/- 165 micrograms/ml in the lavage fluid, which was significantly (P < 0.05) lower than the C5a group (1645 +/- 363 micrograms/ml). Therefore, CRP inhibits the development of neutrophil alveolitis and protein leakage in vivo and inhibits neutrophil chemotaxis in vitro. These data indicate that CRP offers a protective effect in neutrophil-mediated lung injury by reducing neutrophil influx and protein leak.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON H. C., McCARTY M. The occurrence in the rabbit of an acute phase protein analogous to human C reactive protein. J Exp Med. 1951 Jan;93(1):25–36. doi: 10.1084/jem.93.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach B. A., Gewurz H., Osmand A. P. C-reative protein in the rabbit: isolation, characterization and binding affinity to phosphocholine. Immunochemistry. 1977 Mar;14(3):215–219. doi: 10.1016/0019-2791(77)90197-5. [DOI] [PubMed] [Google Scholar]
- Bergh K., Iverson O. J. Intra-assay complement activation may falsely indicate the presence of chemotactic factors in a test plasma or serum. J Immunol Methods. 1989 Sep 29;123(1):147–148. doi: 10.1016/0022-1759(89)90039-2. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Claus D. R., Siegel J., Petras K., Osmand A. P., Gewurz H. Interactions of C-reactive protein with the first component of human complement. J Immunol. 1977 Jul;119(1):187–192. [PubMed] [Google Scholar]
- Cohen A. B., Batra G. K. Bronchoscopy and lung lavage induced bilateral pulmonary neutrophil influx and blood leukocytosis in dogs and monkeys. Am Rev Respir Dis. 1980 Aug;122(2):239–247. doi: 10.1164/arrd.1980.122.2.239. [DOI] [PubMed] [Google Scholar]
- Dobrinich R., Spagnuolo P. J. Binding of C-reactive protein to human neutrophils. Inhibition of respiratory burst activity. Arthritis Rheum. 1991 Aug;34(8):1031–1038. doi: 10.1002/art.1780340813. [DOI] [PubMed] [Google Scholar]
- Du Clos T. W., Mold C., Paterson P. Y., Alroy J., Gewurz H. Localization of C-reactive protein in inflammatory lesions of experimental allergic encephalomyelitis. Clin Exp Immunol. 1981 Mar;43(3):565–573. [PMC free article] [PubMed] [Google Scholar]
- Ganrot P. O., Kindmark C. O. C-reactive protein--a phagocytosis-promoting factor. Scand J Clin Lab Invest. 1969 Oct;24(3):215–219. doi: 10.3109/00365516909080155. [DOI] [PubMed] [Google Scholar]
- Hurlimann J., Thorbecke G. J., Hochwald G. M. The liver as the site of C-reactive protein formation. J Exp Med. 1966 Feb 1;123(2):365–378. doi: 10.1084/jem.123.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUSHNER I., KAPLAN M. H. Studies of acute phase protein. I. An immunohistochemical method for the localization of Cx-reactive protein in rabbits. Association with necrosis in local inflammatory lesions. J Exp Med. 1961 Dec 1;114:961–974. doi: 10.1084/jem.114.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M. H., Volanakis J. E. Interaction of C-reactive protein complexes with the complement system. I. Consumption of human complement associated with the reaction of C-reactive protein with pneumococcal C-polysaccharide and with the choline phosphatides, lecithin and sphingomyelin. J Immunol. 1974 Jun;112(6):2135–2147. [PubMed] [Google Scholar]
- Kazmierowski J. A., Gallin J. I., Reynolds H. Y. Mechanism for the inflammatory response in primate lungs. Demonstration and partial characterization of an alveolar macrophage-derived chemotactic factor with preferential activity for polymorphonuclear leukocytes. J Clin Invest. 1977 Feb;59(2):273–281. doi: 10.1172/JCI108638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kew R. R., Hyers T. M., Webster R. O. Human C-reactive protein inhibits neutrophil chemotaxis in vitro: possible implications for the adult respiratory distress syndrome. J Lab Clin Med. 1990 Mar;115(3):339–345. [PubMed] [Google Scholar]
- Kushner I., Gewurz H., Benson M. D. C-reactive protein and the acute-phase response. J Lab Clin Med. 1981 Jun;97(6):739–749. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larsen G. L., McCarthy K., Webster R. O., Henson J., Henson P. M. A differential effect of C5a and C5a des Arg in the induction of pulmonary inflammation. Am J Pathol. 1980 Jul;100(1):179–192. [PMC free article] [PubMed] [Google Scholar]
- Mortensen R. F., Braun D., Gewurz H. Effects of C-reactive protein on lymphocyte functions. III. Inhibition of antigen-induced lymphocyte stimulation and lymphokine production. Cell Immunol. 1977 Jan;28(1):59–68. doi: 10.1016/s0008-8749(77)80006-3. [DOI] [PubMed] [Google Scholar]
- Mortensen R. F., Duszkiewicz J. A. Mediation of CRP-dependent phagocytosis through mouse macrophage Fc-receptors. J Immunol. 1977 Nov;119(5):1611–1616. [PubMed] [Google Scholar]
- Parish W. E. Studies on vasculitis. VII. C-reactive protein as a substance perpetuating chronic vasculitis. Occurrence in lesions and concentrations in sera. Clin Allergy. 1976 Nov;6(6):543–550. doi: 10.1111/j.1365-2222.1976.tb01939.x. [DOI] [PubMed] [Google Scholar]
- Robbins R. A., Russ W. D., Rasmussen J. K., Clayton M. M. Activation of the complement system in the adult respiratory distress syndrome. Am Rev Respir Dis. 1987 Mar;135(3):651–658. doi: 10.1164/arrd.1987.135.3.651. [DOI] [PubMed] [Google Scholar]
- Robey F. A., Jones K. D., Steinberg A. D. C-reactive protein mediates the solubilization of nuclear DNA by complement in vitro. J Exp Med. 1985 Jun 1;161(6):1344–1356. doi: 10.1084/jem.161.6.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. O., Henson P. M., Henson J., Webster R. O. Lung inflammation induced by complement-derived chemotactic fragments in the alveolus. Lab Invest. 1980 May;42(5):547–558. [PubMed] [Google Scholar]
- Tate R. M., Repine J. E. Neutrophils and the adult respiratory distress syndrome. Am Rev Respir Dis. 1983 Sep;128(3):552–559. doi: 10.1164/arrd.1983.128.3.552. [DOI] [PubMed] [Google Scholar]
- Vigo C. Effect of C-reactive protein on platelet-activating factor-induced platelet aggregation and membrane stabilization. J Biol Chem. 1985 Mar 25;260(6):3418–3422. [PubMed] [Google Scholar]
- Wang C. M., Nguyen N. Y., Yonaha K., Robey F., Liu T. Y. Primary structure of rabbit C-reactive protein. J Biol Chem. 1982 Nov 25;257(22):13610–13615. [PubMed] [Google Scholar]
- Webster R. O., Henson P. M. Rapid micromeasurement of neutrophil exocytosis. Inflammation. 1978 Jun;3(2):129–135. doi: 10.1007/BF00910734. [DOI] [PubMed] [Google Scholar]
- Webster R. O., Hong S. R., Johnston R. B., Jr, Henson P. M. Biologial effects of the human complement fragments C5a and C5ades Arg on neutrophil function. Immunopharmacology. 1980 Jun;2(3):201–219. doi: 10.1016/0162-3109(80)90050-8. [DOI] [PubMed] [Google Scholar]
- Yen-Watson B., Kushner I. Rabbit CRP response to endotoxin administration: dose-response relationship and kinetics. Proc Soc Exp Biol Med. 1974 Sep;146(4):1132–1136. doi: 10.3181/00379727-146-38260. [DOI] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]



