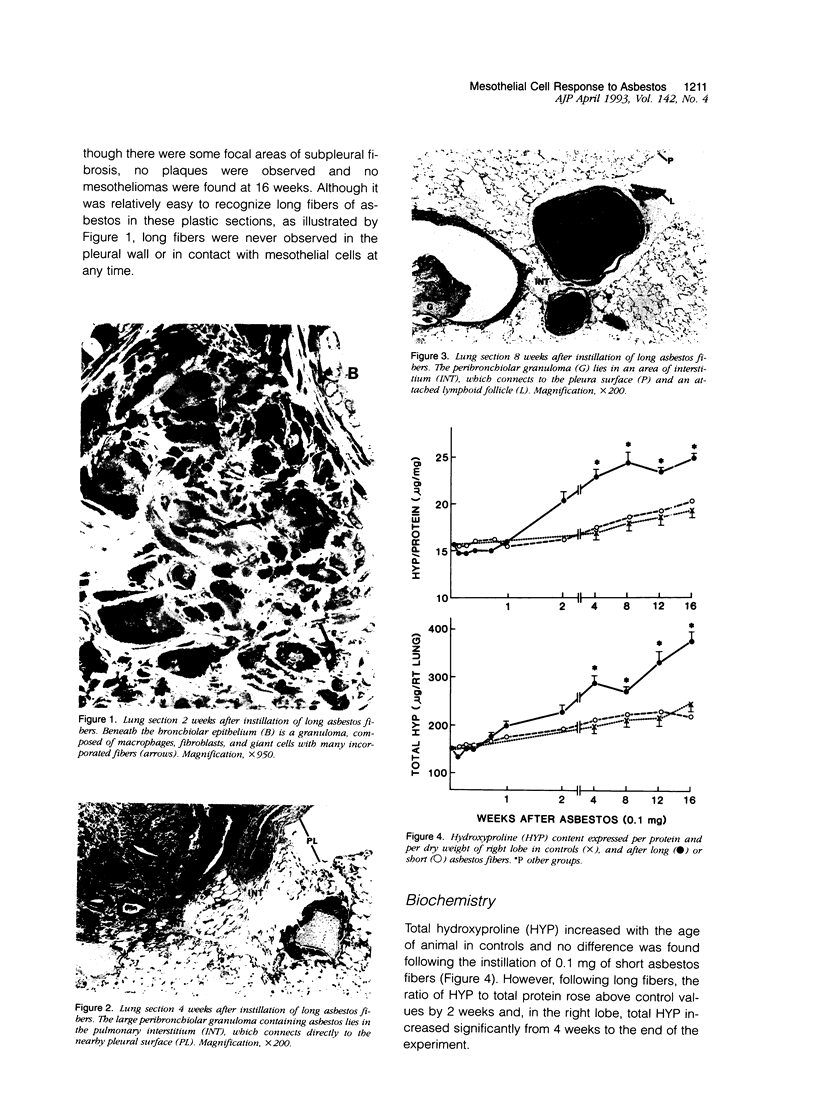
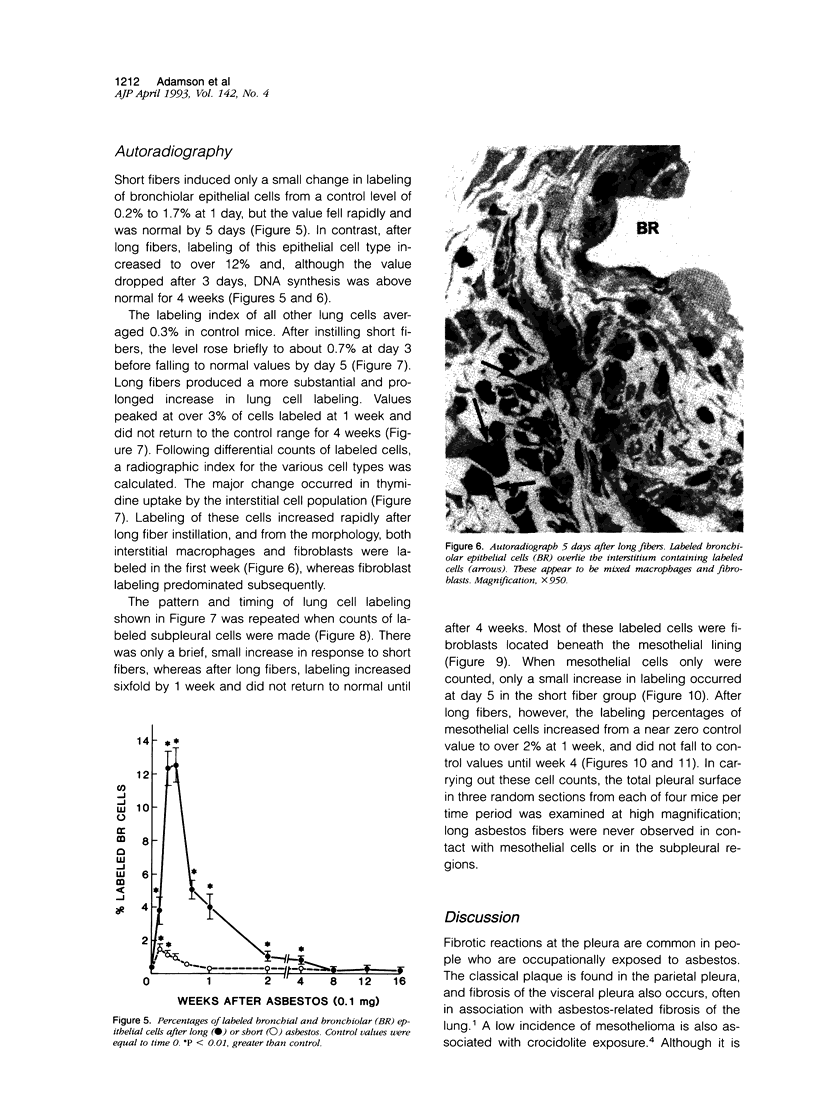
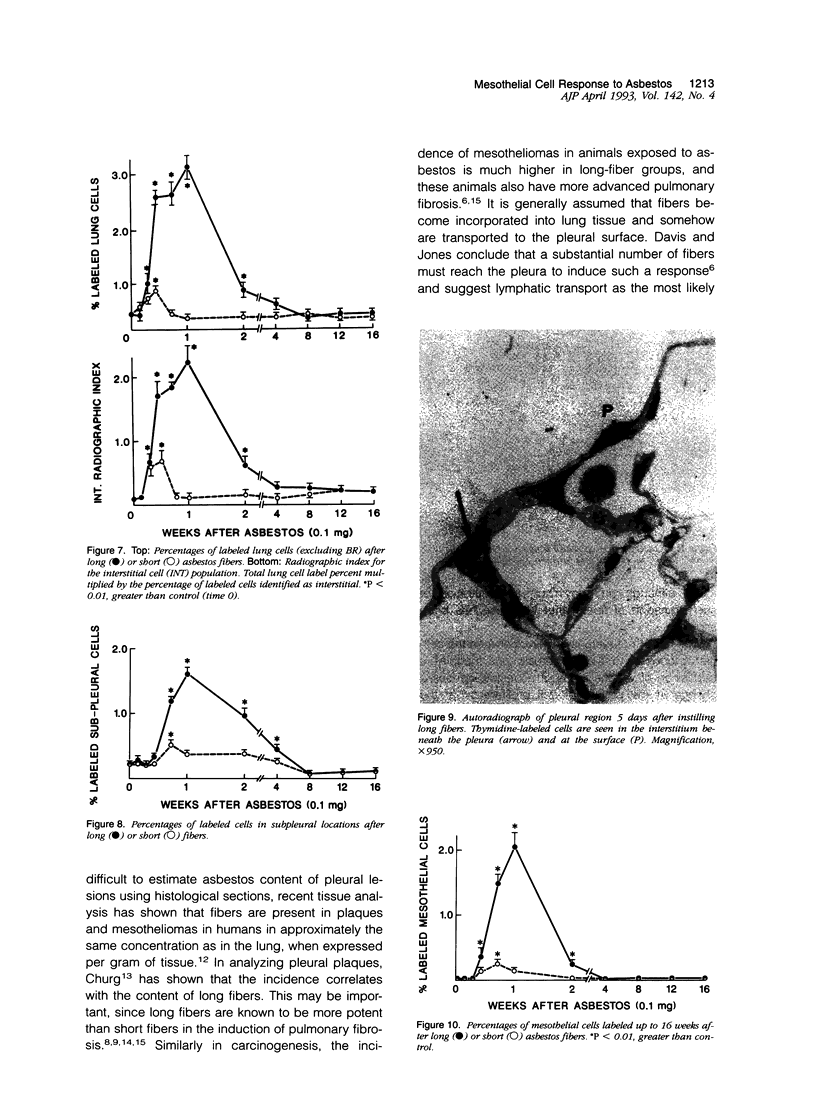
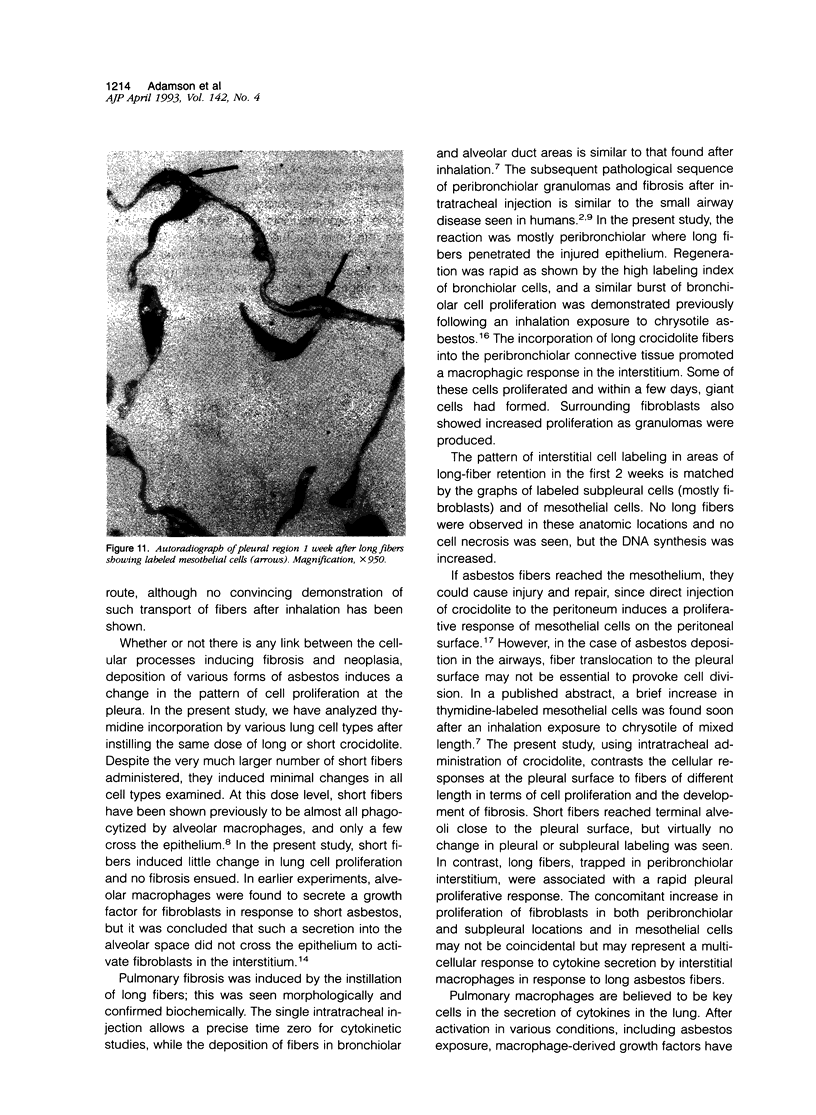
Abstract
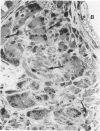
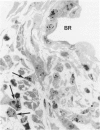
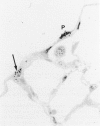
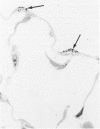
The relationship of asbestos deposition in the lung to subsequent cell proliferation at the pleural surface is not clear. The present study examines DNA synthesis by various pulmonary cells, particularly those at the pleura after intratracheal injection of 0.1 mg crocidolite to mice using: 1) long fibers (> 20 mu), which are deposited in bronchiolar regions and induce fibrosis; 2) short fibers (< 1 mu), which reach alveoli but do not induce fibrosis. Mice also received 2 microCi/g tritiated thymidine 1 hour before death at intervals to 16 weeks. Short fibers induced only a small increase in labeling of bronchiolar epithelial and interstitial cells, which subsided by 5 days, when a small increase in labeled mesothelial and subpleural cells was seen. In contrast, long fibers damaged the bronchiolar epithelium and became incorporated into connective tissue. During regeneration, 12% of cells were labeled at 3 days and labeling was greater than controls to 4 weeks. Increased peribronchiolar labeling of fibroblasts and interstitial macrophages was seen around long fibers, and increased DNA synthesis by mesothelial and subpleural cells was found. Up to 2% of mesothelial cells were labeled 1 week after long fibers compared to near zero in controls. No long fibers were found at the pleura. Activation of interstitial macrophages in response to long crocidolite fibers is associated with fibroblast proliferation. It is now suggested that mesothelial cells may also be stimulated by cytokines from activated interstitial macrophages that diffuse across the interstitium, without requiring actual fiber translocation to the pleura.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson I. Y., Bowden D. H. Pulmonary reaction to long and short asbestos fibers is independent of fibroblast growth factor production by alveolar macrophages. Am J Pathol. 1990 Sep;137(3):523–529. [PMC free article] [PubMed] [Google Scholar]
- Adamson I. Y., Bowden D. H. Response of mouse lung to crocidolite asbestos. 1. Minimal fibrotic reaction to short fibres. J Pathol. 1987 Jun;152(2):99–107. doi: 10.1002/path.1711520206. [DOI] [PubMed] [Google Scholar]
- Adamson I. Y., Bowden D. H. Response of mouse lung to crocidolite asbestos. 2. Pulmonary fibrosis after long fibres. J Pathol. 1987 Jun;152(2):109–117. doi: 10.1002/path.1711520207. [DOI] [PubMed] [Google Scholar]
- Adamson I. Y., Letourneau H. L., Bowden D. H. Comparison of alveolar and interstitial macrophages in fibroblast stimulation after silica and long or short asbestos. Lab Invest. 1991 Mar;64(3):339–344. [PubMed] [Google Scholar]
- Bissonnette E., Rola-Pleszczynski M. Pulmonary inflammation and fibrosis in a murine model of asbestosis and silicosis. Possible role of tumor necrosis factor. Inflammation. 1989 Jun;13(3):329–339. doi: 10.1007/BF00914399. [DOI] [PubMed] [Google Scholar]
- Bitterman P. B., Adelberg S., Crystal R. G. Mechanisms of pulmonary fibrosis. Spontaneous release of the alveolar macrophage-derived growth factor in the interstitial lung disorders. J Clin Invest. 1983 Nov;72(5):1801–1813. doi: 10.1172/JCI111140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden D. H., Adamson I. Y. Bronchiolar and alveolar lesions in the pathogenesis of crocidolite-induced pulmonary fibrosis in mice. J Pathol. 1985 Dec;147(4):257–267. doi: 10.1002/path.1711470404. [DOI] [PubMed] [Google Scholar]
- Brody A. R., Overby L. H. Incorporation of tritiated thymidine by epithelial and interstitial cells in bronchiolar-alveolar regions of asbestos-exposed rats. Am J Pathol. 1989 Jan;134(1):133–140. [PMC free article] [PubMed] [Google Scholar]
- Churg A. Asbestos fibers and pleural plaques in a general autopsy population. Am J Pathol. 1982 Oct;109(1):88–96. [PMC free article] [PubMed] [Google Scholar]
- Craighead J. E., Mossman B. T. The pathogenesis of asbestos-associated diseases. N Engl J Med. 1982 Jun 17;306(24):1446–1455. doi: 10.1056/NEJM198206173062403. [DOI] [PubMed] [Google Scholar]
- Davis J. M., Addison J., Bolton R. E., Donaldson K., Jones A. D., Smith T. The pathogenicity of long versus short fibre samples of amosite asbestos administered to rats by inhalation and intraperitoneal injection. Br J Exp Pathol. 1986 Jun;67(3):415–430. [PMC free article] [PubMed] [Google Scholar]
- Davis J. M., Jones A. D. Comparisons of the pathogenicity of long and short fibres of chrysotile asbestos in rats. Br J Exp Pathol. 1988 Oct;69(5):717–737. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lemaire I., Beaudoin H., Massé S., Grondin C. Alveolar macrophage stimulation of lung fibroblast growth in asbestos-induced pulmonary fibrosis. Am J Pathol. 1986 Feb;122(2):205–211. [PMC free article] [PubMed] [Google Scholar]
- Mangum J. B., Everitt J. I., Bonner J. C., Moore L. R., Brody A. R. Co-culture of primary pulmonary cells to model alveolar injury and translocation of proteins. In Vitro Cell Dev Biol. 1990 Dec;26(12):1135–1143. doi: 10.1007/BF02623690. [DOI] [PubMed] [Google Scholar]
- Moalli P. A., MacDonald J. L., Goodglick L. A., Kane A. B. Acute injury and regeneration of the mesothelium in response to asbestos fibers. Am J Pathol. 1987 Sep;128(3):426–445. [PMC free article] [PubMed] [Google Scholar]
- Mossman B. T., Gee J. B. Asbestos-related diseases. N Engl J Med. 1989 Jun 29;320(26):1721–1730. doi: 10.1056/NEJM198906293202604. [DOI] [PubMed] [Google Scholar]
- Schapira R. M., Osornio-Vargas A. R., Brody A. R. Inorganic particles induce secretion of a macrophage homologue of platelet-derived growth factor in a density-and time-dependent manner in vitro. Exp Lung Res. 1991 Nov-Dec;17(6):1011–1024. doi: 10.3109/01902149109064332. [DOI] [PubMed] [Google Scholar]
- Stanton M. F., Wrench C. Mechanisms of mesothelioma induction with asbestos and fibrous glass. J Natl Cancer Inst. 1972 Mar;48(3):797–821. [PubMed] [Google Scholar]
- Walker C., Bermudez E., Stewart W., Bonner J., Molloy C. J., Everitt J. Characterization of platelet-derived growth factor and platelet-derived growth factor receptor expression in asbestos-induced rat mesothelioma. Cancer Res. 1992 Jan 15;52(2):301–306. [PubMed] [Google Scholar]
- Wright J. L., Churg A. Morphology of small-airway lesions in patients with asbestos exposure. Hum Pathol. 1984 Jan;15(1):68–74. doi: 10.1016/s0046-8177(84)80332-9. [DOI] [PubMed] [Google Scholar]