Abstract
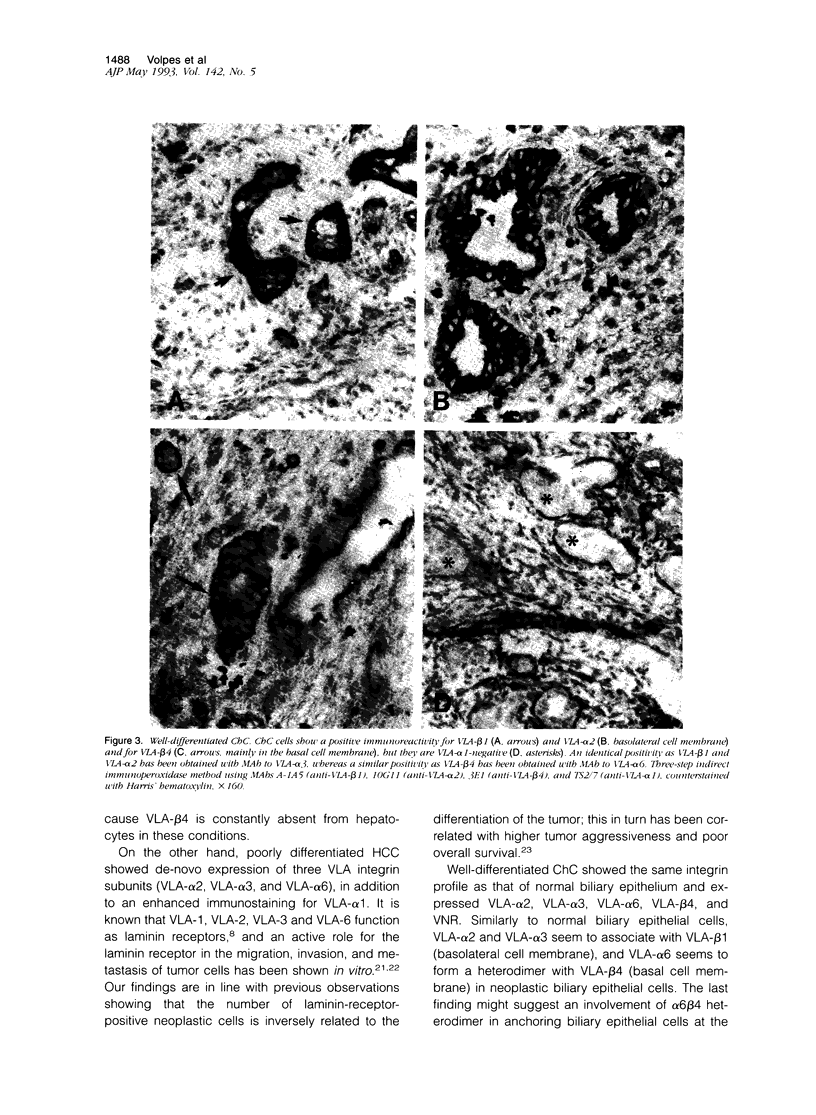
This study analyzed new cell lineage markers for the differential diagnosis between hepatocellular carcinoma (HCC) and cholangiocarcinoma (ChC), as well as the potential pathways of cell-cell and cell-extracellular matrix interactions of neoplastic liver cells during tumor spread and invasion, by comparing the expression of (VLA) integrins, vitronectin receptor, and neural cell adhesion molecule in normal, inflamed, and neoplastic human liver biopsies. All cases of liver cell adenoma and well-differentiated HCC expressed the same set of integrins as observed in normal liver tissue, i.e., VLA-alpha 1 and VLA-beta 1. Poorly differentiated HCC also expressed VLA-alpha 1 and VLA-beta 1, but in addition de-novo expressed VLA-alpha 2, VLA-alpha 3, VLA-alpha 6 and vitronectin receptor. All cases of well-differentiated ChC expressed an identical integrin immunoprofile as observed in normal bile duct epithelium, i.e., VLA-alpha 2, VLA-alpha 3, VLA-alpha 6, VLA-beta 4 and vitronectin receptor, whereas poorly differentiated ChC showed a markedly decreased expression of these integrin subunits. VLA-alpha 1 was constantly absent from all cases of ChC, whereas VLA-beta 4 was never expressed by HCC. Neural cell adhesion molecule, exclusively expressed by proliferating reactive bile ductules in cholestatic and regenerating liver, was constantly absent from both malignant neoplasms. In conclusion, the integrin make up of various liver tumors closely follows that of their normal counterparts. Differences in integrin receptor expression vary according to the cellular origin of the tumors and are associated with a poor differentiation. Our findings suggest that immunohistochemical staining for VLA-alpha 1 and VLA-beta 4 integrin subunits, which highlight the cellular phenotype of the two neoplasms, might be a helpful tool in the differential diagnosis between HCC and ChC.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bissell M. J., Hall H. G., Parry G. How does the extracellular matrix direct gene expression? J Theor Biol. 1982 Nov 7;99(1):31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- Brumm C., Schulze C., Charels K., Morohoshi T., Klöppel G. The significance of alpha-fetoprotein and other tumour markers in differential immunocytochemistry of primary liver tumours. Histopathology. 1989 May;14(5):503–513. doi: 10.1111/j.1365-2559.1989.tb02186.x. [DOI] [PubMed] [Google Scholar]
- Burridge K., Fath K., Kelly T., Nuckolls G., Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Carter W. G., Kaur P., Gil S. G., Gahr P. J., Wayner E. A. Distinct functions for integrins alpha 3 beta 1 in focal adhesions and alpha 6 beta 4/bullous pemphigoid antigen in a new stable anchoring contact (SAC) of keratinocytes: relation to hemidesmosomes. J Cell Biol. 1990 Dec;111(6 Pt 2):3141–3154. doi: 10.1083/jcb.111.6.3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter W. G., Wayner E. A., Bouchard T. S., Kaur P. The role of integrins alpha 2 beta 1 and alpha 3 beta 1 in cell-cell and cell-substrate adhesion of human epidermal cells. J Cell Biol. 1990 Apr;110(4):1387–1404. doi: 10.1083/jcb.110.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett J., Harris A. L. Cell adhesion molecules and cancer. Curr Opin Oncol. 1992 Feb;4(1):142–148. doi: 10.1097/00001622-199202000-00019. [DOI] [PubMed] [Google Scholar]
- Fradet Y., Cordon-Cardo C., Thomson T., Daly M. E., Whitmore W. F., Jr, Lloyd K. O., Melamed M. R., Old L. J. Cell surface antigens of human bladder cancer defined by mouse monoclonal antibodies. Proc Natl Acad Sci U S A. 1984 Jan;81(1):224–228. doi: 10.1073/pnas.81.1.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganjei P., Nadji M., Albores-Saavedra J., Morales A. R. Histologic markers in primary and metastatic tumors of the liver. Cancer. 1988 Nov 1;62(9):1994–1998. doi: 10.1002/1097-0142(19881101)62:9<1994::aid-cncr2820620920>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Giltay J. C., Brinkman H. J., Modderman P. W., von dem Borne A. E., van Mourik J. A. Human vascular endothelial cells express a membrane protein complex immunochemically indistinguishable from the platelet VLA-2 (glycoprotein Ia-IIa) complex. Blood. 1989 Apr;73(5):1235–1241. [PubMed] [Google Scholar]
- Grigioni W. F., Garbisa S., D'Errico A., Baccarini P., Stetler-Stevenson W. G., Liotta L. A., Mancini A. M. Evaluation of hepatocellular carcinoma aggressiveness by a panel of extracellular matrix antigens. Am J Pathol. 1991 Mar;138(3):647–654. [PMC free article] [PubMed] [Google Scholar]
- Hall P. A., Coates P., Lemoine N. R., Horton M. A. Characterization of integrin chains in normal and neoplastic human pancreas. J Pathol. 1991 Sep;165(1):33–41. doi: 10.1002/path.1711650107. [DOI] [PubMed] [Google Scholar]
- Hemler M. E., Sanchez-Madrid F., Flotte T. J., Krensky A. M., Burakoff S. J., Bhan A. K., Springer T. A., Strominger J. L. Glycoproteins of 210,000 and 130,000 m.w. on activated T cells: cell distribution and antigenic relation to components on resting cells and T cell lines. J Immunol. 1984 Jun;132(6):3011–3018. [PubMed] [Google Scholar]
- Hemler M. E., Ware C. F., Strominger J. L. Characterization of a novel differentiation antigen complex recognize by a monoclonal antibody (A-1A5): unique activation-specific molecular forms on stimulated T cells. J Immunol. 1983 Jul;131(1):334–340. [PubMed] [Google Scholar]
- Humphries M. J. The molecular basis and specificity of integrin-ligand interactions. J Cell Sci. 1990 Dec;97(Pt 4):585–592. doi: 10.1242/jcs.97.4.585. [DOI] [PubMed] [Google Scholar]
- Koretz K., Schlag P., Boumsell L., Möller P. Expression of VLA-alpha 2, VLA-alpha 6, and VLA-beta 1 chains in normal mucosa and adenomas of the colon, and in colon carcinomas and their liver metastases. Am J Pathol. 1991 Mar;138(3):741–750. [PMC free article] [PubMed] [Google Scholar]
- Koukoulis G. K., Virtanen I., Korhonen M., Laitinen L., Quaranta V., Gould V. E. Immunohistochemical localization of integrins in the normal, hyperplastic, and neoplastic breast. Correlations with their functions as receptors and cell adhesion molecules. Am J Pathol. 1991 Oct;139(4):787–799. [PMC free article] [PubMed] [Google Scholar]
- Lai Y. S., Thung S. N., Gerber M. A., Chen M. L., Schaffner F. Expression of cytokeratins in normal and diseased livers and in primary liver carcinomas. Arch Pathol Lab Med. 1989 Feb;113(2):134–138. [PubMed] [Google Scholar]
- Larjava H., Peltonen J., Akiyama S. K., Yamada S. S., Gralnick H. R., Uitto J., Yamada K. M. Novel function for beta 1 integrins in keratinocyte cell-cell interactions. J Cell Biol. 1990 Mar;110(3):803–815. doi: 10.1083/jcb.110.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta L. A., Rao C. N., Barsky S. H. Tumor invasion and the extracellular matrix. Lab Invest. 1983 Dec;49(6):636–649. [PubMed] [Google Scholar]
- Nakajima T., Kondo Y. Well-differentiated cholangiocarcinoma: diagnostic significance of morphologic and immunohistochemical parameters. Am J Surg Pathol. 1989 Jul;13(7):569–573. [PubMed] [Google Scholar]
- Pastolero G. C., Wakabayashi T., Oka T., Mori S. Tissue polypeptide antigen--a marker antigen differentiating cholangiolar tumors from other hepatic tumors. Am J Clin Pathol. 1987 Feb;87(2):168–173. doi: 10.1093/ajcp/87.2.168. [DOI] [PubMed] [Google Scholar]
- Pignatelli M., Hanby A. M., Stamp G. W. Low expression of beta 1, alpha 2 and alpha 3 subunits of VLA integrins in malignant mammary tumours. J Pathol. 1991 Sep;165(1):25–32. doi: 10.1002/path.1711650106. [DOI] [PubMed] [Google Scholar]
- Roskams T., De Vos R., van den Oord J. J., Desmet V. Cells with neuroendocrine features in regenerating human liver. APMIS Suppl. 1991;23:32–39. [PubMed] [Google Scholar]
- Roskams T., van den Oord J. J., De Vos R., Desmet V. J. Neuroendocrine features of reactive bile ductules in cholestatic liver disease. Am J Pathol. 1990 Nov;137(5):1019–1025. [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E., Giancotti F. G. Integrins and tumor cell dissemination. Cancer Cells. 1989 Dec;1(4):119–126. [PubMed] [Google Scholar]
- Ruoslahti E. Integrins. J Clin Invest. 1991 Jan;87(1):1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell S., Dunsford H. A. Evidence for the stem cell origin of hepatocellular carcinoma and cholangiocarcinoma. Am J Pathol. 1989 Jun;134(6):1347–1363. [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg A., Janssen H., Hogervorst F., Calafat J., Hilgers J. A complex of platelet glycoproteins Ic and IIa identified by a rat monoclonal antibody. J Biol Chem. 1987 Jul 25;262(21):10376–10383. [PubMed] [Google Scholar]
- Stamp G. W., Pignatelli M. Distribution of beta 1, alpha 1, alpha 2 and alpha 3 integrin chains in basal cell carcinomas. J Pathol. 1991 Apr;163(4):307–313. doi: 10.1002/path.1711630407. [DOI] [PubMed] [Google Scholar]
- Tamura R. N., Rozzo C., Starr L., Chambers J., Reichardt L. F., Cooper H. M., Quaranta V. Epithelial integrin alpha 6 beta 4: complete primary structure of alpha 6 and variant forms of beta 4. J Cell Biol. 1990 Oct;111(4):1593–1604. doi: 10.1083/jcb.111.4.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova V. P., Rao C. N., Kalebic T., Margulies I. M., Liotta L. A. Laminin receptor on human breast carcinoma cells. Proc Natl Acad Sci U S A. 1983 Jan;80(2):444–448. doi: 10.1073/pnas.80.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eyken P., Sciot R., Desmet V. J. A cytokeratin immunohistochemical study of cholestatic liver disease: evidence that hepatocytes can express 'bile duct-type' cytokeratins. Histopathology. 1989 Aug;15(2):125–135. doi: 10.1111/j.1365-2559.1989.tb03060.x. [DOI] [PubMed] [Google Scholar]
- Volpes R., van den Oord J. J., Desmet V. J. Distribution of the VLA family of integrins in normal and pathological human liver tissue. Gastroenterology. 1991 Jul;101(1):200–206. doi: 10.1016/0016-5085(91)90478-4. [DOI] [PubMed] [Google Scholar]
- Wewer U. M., Taraboletti G., Sobel M. E., Albrechtsen R., Liotta L. A. Role of laminin receptor in tumor cell migration. Cancer Res. 1987 Nov 1;47(21):5691–5698. [PubMed] [Google Scholar]
- Zutter M. M., Mazoujian G., Santoro S. A. Decreased expression of integrin adhesive protein receptors in adenocarcinoma of the breast. Am J Pathol. 1990 Oct;137(4):863–870. [PMC free article] [PubMed] [Google Scholar]