Abstract
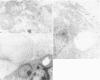
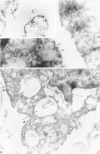
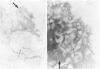
Prematurely born infants can develop the neonatal respiratory distress syndrome (RDS) because of a deficiency of pulmonary surfactant. This lipoprotein complex synthesized by type II pneumocytes has different ultrastructural forms--intra- and extracellular lamellar bodies, which within the alveoli are transformed into tubular myelin, and this in turn gives rise to the surface monolayer, the functionally active form of surfactant. We have previously shown that at autopsy RDS lungs lack tubular myelin and have decreased immunoreactivity for antisera to surfactant protein A (SP-A), an important component of tubular myelin. Therefore, we proposed a role for SP-A in the conversion of lamellar bodies to tubular myelin and in the pathogenesis of RDS. To explore this possibility further, we compared in 14 RDS and 14 control lungs the distribution of SP-A in ultrathin sections, using affinity-purified rabbit anti-human-SP-A IgG and goat anti-rabbit IgG-conjugated with 10 nm colloidal gold particles. In controls, gold label was present in lamellar bodies, endoplasmic reticulum, on the cytoplasmic membrane of type II cells, and on lamellar bodies and tubular myelin either within alveoli or macrophages. In RDS lungs, reduced label was present in the same intracellular compartments and organelles, except in tubular myelin, which is absent. It is postulated that if SP-A is indeed necessary for the conversion of lamellar bodies to tubular myelin, in RDS either there is a deficiency of adequate amounts of functional SP-A or some other important component of surfactant is missing.
Full text
PDF
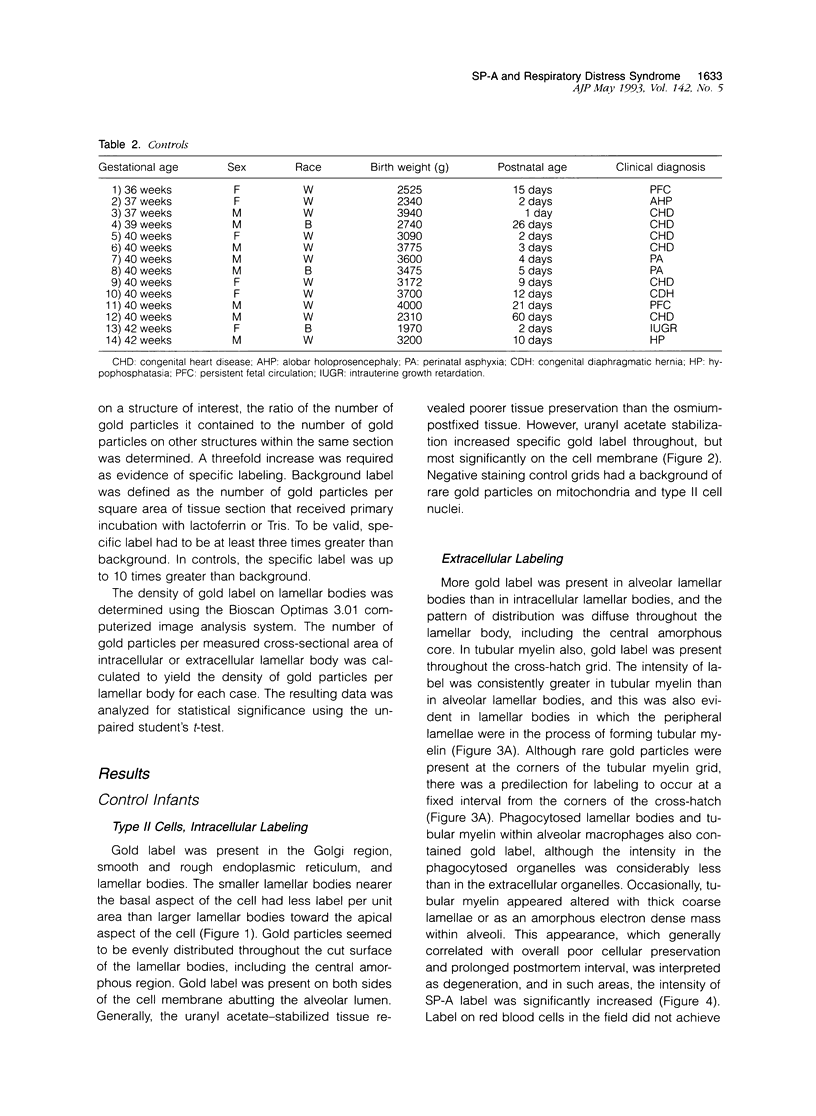
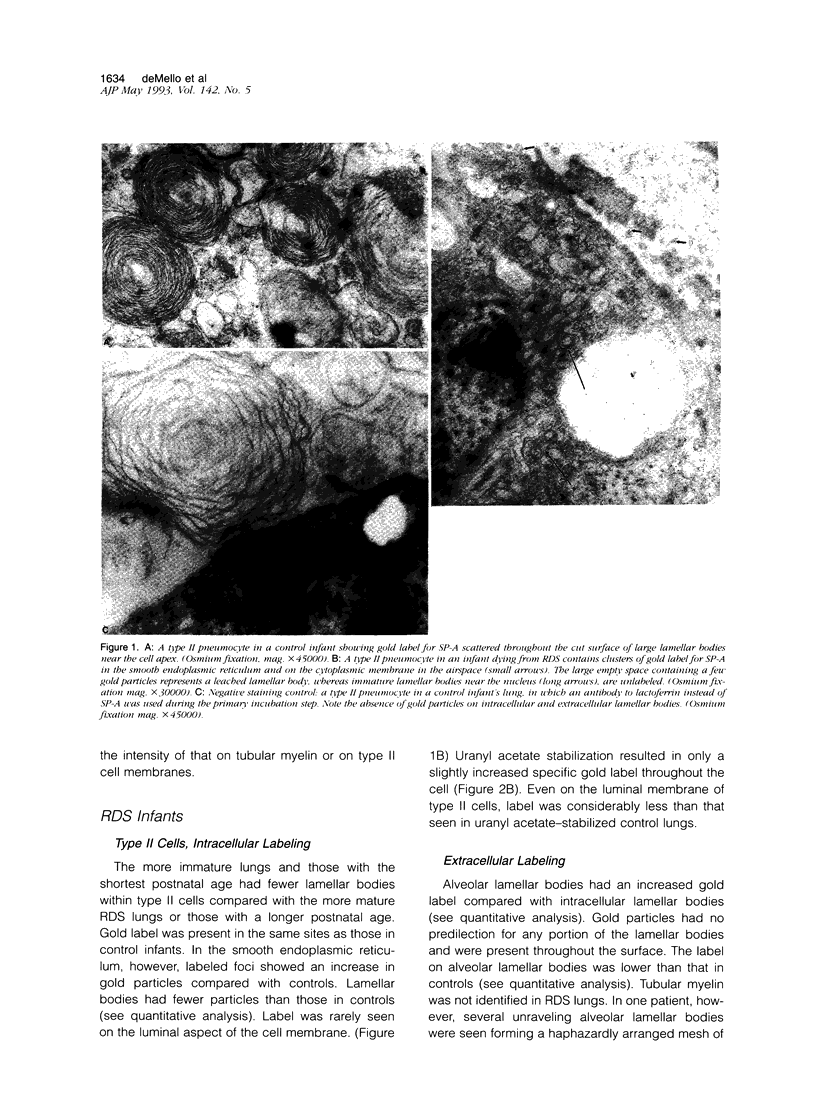

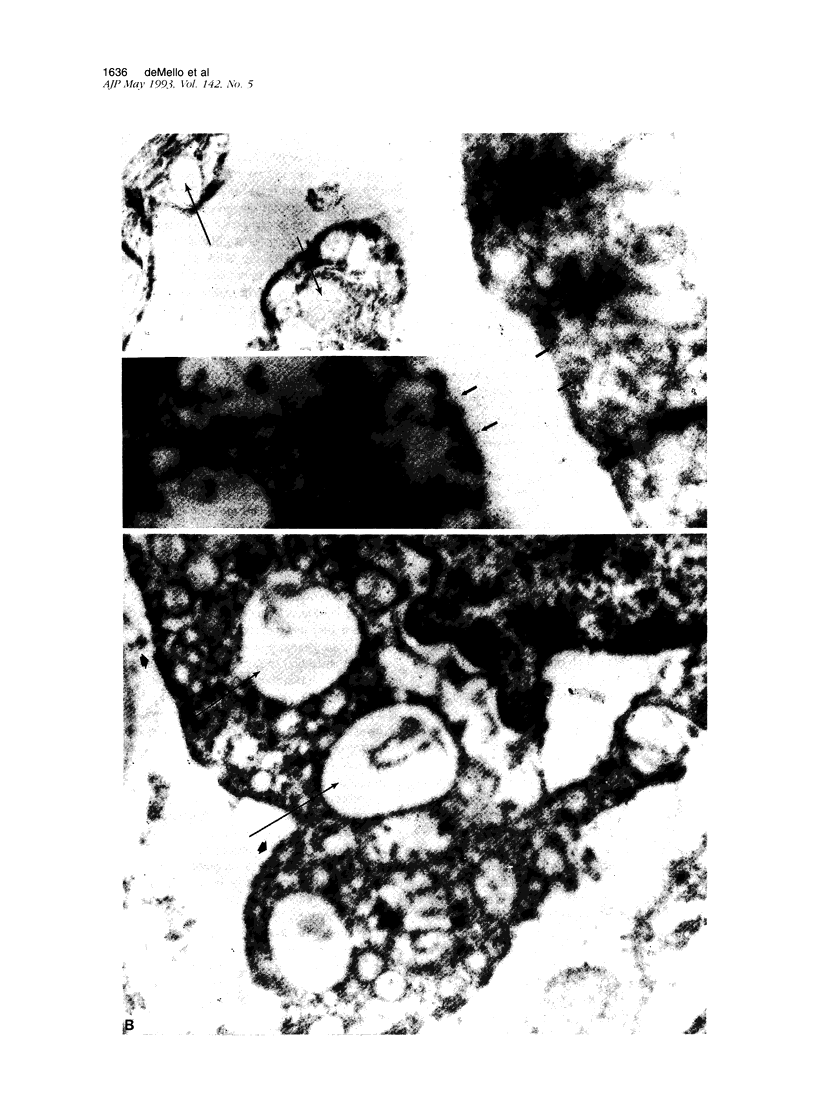
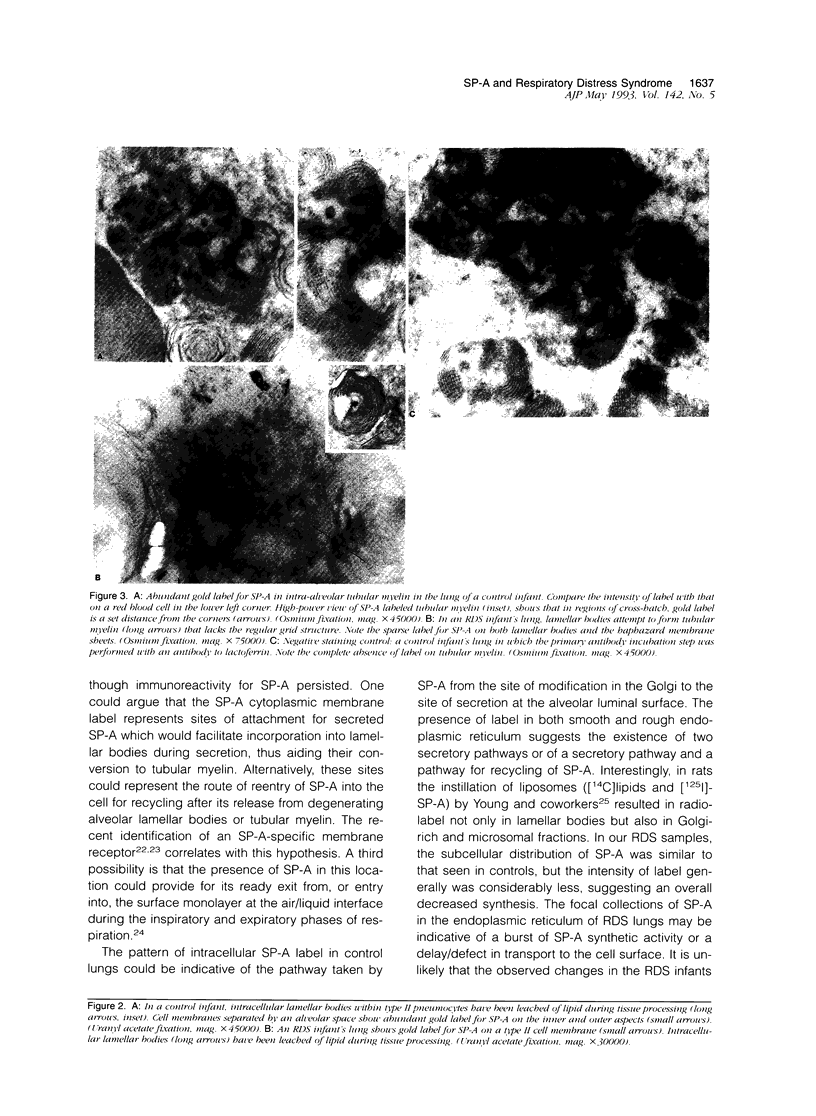
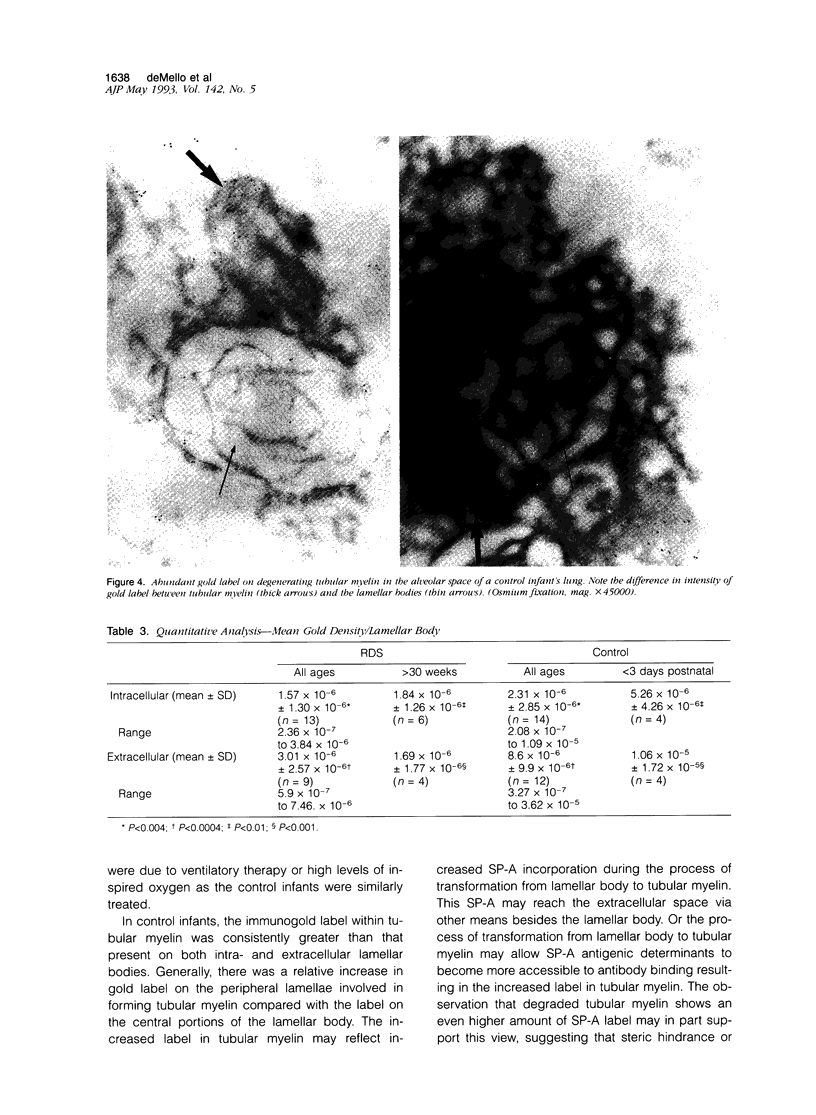


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVERY M. E., MEAD J. Surface properties in relation to atelectasis and hyaline membrane disease. AMA J Dis Child. 1959 May;97(5 Pt 1):517–523. doi: 10.1001/archpedi.1959.02070010519001. [DOI] [PubMed] [Google Scholar]
- Balis J. U., Paterson J. F., Paciga J. E., Haller E. M., Shelley S. A. Distribution and subcellular localization of surfactant-associated glycoproteins in human lung. Lab Invest. 1985 Jun;52(6):657–669. [PubMed] [Google Scholar]
- Berryman M. A., Rodewald R. D. An enhanced method for post-embedding immunocytochemical staining which preserves cell membranes. J Histochem Cytochem. 1990 Feb;38(2):159–170. doi: 10.1177/38.2.1688894. [DOI] [PubMed] [Google Scholar]
- CLEMENTS J. A. Surface tension of lung extracts. Proc Soc Exp Biol Med. 1957 May;95(1):170–172. doi: 10.3181/00379727-95-23156. [DOI] [PubMed] [Google Scholar]
- Coalson J. J., Winter V. T., Martin H. M., King R. J. Colloidal gold immunoultrastructural localization of rat surfactant. Am Rev Respir Dis. 1986 Feb;133(2):230–237. doi: 10.1164/arrd.1986.133.2.230. [DOI] [PubMed] [Google Scholar]
- Floros J. Sixty years of surfactant research. Am J Physiol. 1990 Apr;258(4 Pt 1):L238–L240. doi: 10.1152/ajplung.1990.258.4.L238. [DOI] [PubMed] [Google Scholar]
- Froh D., Ballard P. L., Williams M. C., Gonzales J., Goerke J., Odom M. W., Gonzales L. W. Lamellar bodies of cultured human fetal lung: content of surfactant protein A (SP-A), surface film formation and structural transformation in vitro. Biochim Biophys Acta. 1990 Apr 9;1052(1):78–89. doi: 10.1016/0167-4889(90)90060-q. [DOI] [PubMed] [Google Scholar]
- Groniowski J. A. Fine structural basis of pulmonary surfactant. Int Rev Exp Pathol. 1983;25:183–238. [PubMed] [Google Scholar]
- Groniowski J. Presumable role of surface glycoprotein of alveolar pneumonocytes in transformation of lamellar bodies into tubular myelin. Acta Med Pol. 1979;20(4):393–396. [PubMed] [Google Scholar]
- Groniowski J., Walski M. Further studies on the tubular myelin structure of extracellular alveolar lining layer. Acta Med Pol. 1979;20(1):15–16. [PubMed] [Google Scholar]
- Kikkawa Y. Morphology of alveolar lining layer. Anat Rec. 1970 Aug;167(4):389–400. doi: 10.1002/ar.1091670403. [DOI] [PubMed] [Google Scholar]
- King R. J. The surfactant system of the lung. Fed Proc. 1974 Nov;33(11):2238–2247. [PubMed] [Google Scholar]
- Phelps D. S., Floros J. Localization of pulmonary surfactant proteins using immunohistochemistry and tissue in situ hybridization. Exp Lung Res. 1991 Nov-Dec;17(6):985–995. doi: 10.3109/01902149109064330. [DOI] [PubMed] [Google Scholar]
- Phelps D. S., Floros J. Localization of surfactant protein synthesis in human lung by in situ hybridization. Am Rev Respir Dis. 1988 Apr;137(4):939–942. doi: 10.1164/ajrccm/137.4.939. [DOI] [PubMed] [Google Scholar]
- Phelps D. S., Floros J., Taeusch H. W., Jr Post-translational modification of the major human surfactant-associated proteins. Biochem J. 1986 Jul 15;237(2):373–377. doi: 10.1042/bj2370373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps D. S., Harding H. P. Immunohistochemical localization of a low molecular weight surfactant-associated protein in human lung. J Histochem Cytochem. 1987 Nov;35(11):1339–1342. doi: 10.1177/35.11.3309049. [DOI] [PubMed] [Google Scholar]
- Possmayer F. A proposed nomenclature for pulmonary surfactant-associated proteins. Am Rev Respir Dis. 1988 Oct;138(4):990–998. doi: 10.1164/ajrccm/138.4.990. [DOI] [PubMed] [Google Scholar]
- Ryan R. M., Morris R. E., Rice W. R., Ciraolo G., Whitsett J. A. Binding and uptake of pulmonary surfactant protein (SP-A) by pulmonary type II epithelial cells. J Histochem Cytochem. 1989 Apr;37(4):429–440. doi: 10.1177/37.4.2926121. [DOI] [PubMed] [Google Scholar]
- Sorokin S P. A morphologic and cytochemical study on the great alveolar cell. J Histochem Cytochem. 1966 Dec;14(12):884–897. doi: 10.1177/14.12.884. [DOI] [PubMed] [Google Scholar]
- Sueishi K., Tanaka K., Oda T. Immunoultrastructural study of surfactant system. Distribution of specific protein of surface active material in rabbit lung. Lab Invest. 1977 Aug;37(2):136–142. [PubMed] [Google Scholar]
- Suzuki Y., Fujita Y., Kogishi K. Reconstitution of tubular myelin from synthetic lipids and proteins associated with pig pulmonary surfactant. Am Rev Respir Dis. 1989 Jul;140(1):75–81. doi: 10.1164/ajrccm/140.1.75. [DOI] [PubMed] [Google Scholar]
- Voorhout W. F., Veenendaal T., Haagsman H. P., Verkleij A. J., van Golde L. M., Geuze H. J. Surfactant protein A is localized at the corners of the pulmonary tubular myelin lattice. J Histochem Cytochem. 1991 Oct;39(10):1331–1336. doi: 10.1177/39.10.1940306. [DOI] [PubMed] [Google Scholar]
- Williams M. C., Hawgood S., Hamilton R. L. Changes in lipid structure produced by surfactant proteins SP-A, SP-B, and SP-C. Am J Respir Cell Mol Biol. 1991 Jul;5(1):41–50. doi: 10.1165/ajrcmb/5.1.41. [DOI] [PubMed] [Google Scholar]
- Young S. L., Wright J. R., Clements J. A. Cellular uptake and processing of surfactant lipids and apoprotein SP-A by rat lung. J Appl Physiol (1985) 1989 Mar;66(3):1336–1342. doi: 10.1152/jappl.1989.66.3.1336. [DOI] [PubMed] [Google Scholar]
- deMello D. E., Chi E. Y., Doo E., Lagunoff D. Absence of tubular myelin in lungs of infants dying with hyaline membrane disease. Am J Pathol. 1987 Apr;127(1):131–139. [PMC free article] [PubMed] [Google Scholar]
- deMello D. E., Phelps D. S., Patel G., Floros J., Lagunoff D. Expression of the 35kDa and low molecular weight surfactant-associated proteins in the lungs of infants dying with respiratory distress syndrome. Am J Pathol. 1989 Jun;134(6):1285–1293. [PMC free article] [PubMed] [Google Scholar]