Abstract
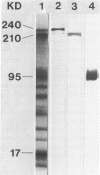
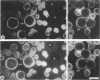
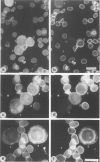
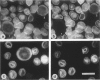
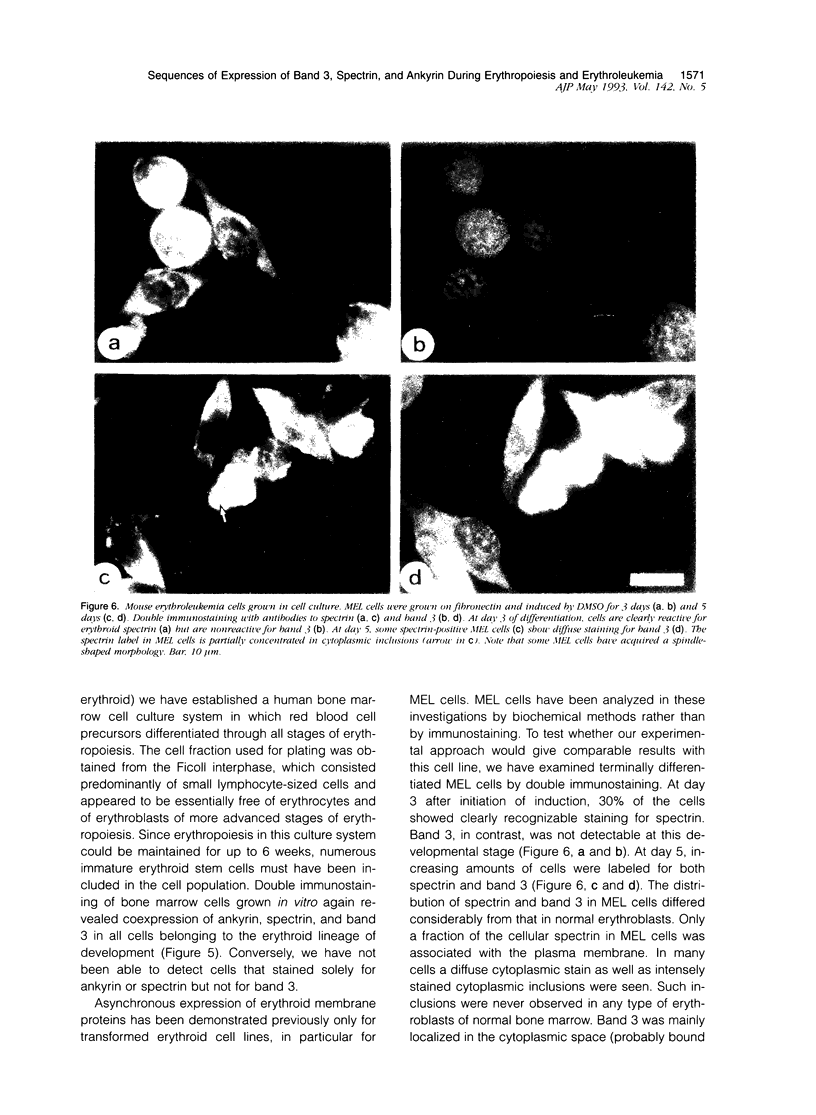
Expression of the erythrocyte anion exchanger band 3, and ankyrin and spectrin, two cytoskeletal proteins of the red blood cell membrane, was studied by immunofluorescence using: 1) smears of human bone marrow from healthy donors and from a patient with erythroleukemia, 2) human red blood cell precursors grown in cell culture, and 3) murine erythroleukemia cells grown in cell culture. Double immunostaining with antibodies to band 3 in combination with spectrin or ankyrin revealed that these proteins become expressed synchronously during normal human erythropoiesis. In contrast, both murine erythroleukemia cells (induced by fibronectin and dimethyl sulfoxide to differentiate in vitro) and erythroblasts from a patient suffering from erythroleukemia displayed distinct asynchronicity in expression of these proteins, ie, ankyrin and spectrin were synthesized first, followed by band 3 at a later stage of erythroid development. After the onset of band 3 expression in human erythroleukemia cells, an increase of membrane-associated fluorescence was detectable for both ankyrin and spectrin, supporting the general view that band 3 promotes assembly of the membrane cytoskeleton. These findings indicate that the current concept of a sequential expression of spectrin/ankyrin and band 3 is valid only for erythroleukemia cells or transformed erythropoietic cell lines but does not occur in normal erythropoiesis, during which these proteins become expressed simultaneously.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett V. The membrane skeleton of human erythrocytes and its implications for more complex cells. Annu Rev Biochem. 1985;54:273–304. doi: 10.1146/annurev.bi.54.070185.001421. [DOI] [PubMed] [Google Scholar]
- Cox J. V., Stack J. H., Lazarides E. Erythroid anion transporter assembly is mediated by a developmentally regulated recruitment onto a preassembled membrane cytoskeleton. J Cell Biol. 1987 Sep;105(3):1405–1416. doi: 10.1083/jcb.105.3.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen H., Bach R., Emery R. Induction of spectrin in erythroleukemic cells transformed by Friend virus. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3898–3902. doi: 10.1073/pnas.74.9.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney J., Glenney P. Co-expression of spectrin and fodrin in Friend erythroleukemic cells treated with DMSO. Exp Cell Res. 1984 May;152(1):15–21. doi: 10.1016/0014-4827(84)90225-8. [DOI] [PubMed] [Google Scholar]
- Koury M. J., Bondurant M. C., Atkinson J. B. Erythropoietin control of terminal erythroid differentiation: maintenance of cell viability, production of hemoglobin, and development of the erythrocyte membrane. Blood Cells. 1987;13(1-2):217–226. [PubMed] [Google Scholar]
- Koury M. J., Bondurant M. C., Mueller T. J. The role of erythropoietin in the production of principal erythrocyte proteins other than hemoglobin during terminal erythroid differentiation. J Cell Physiol. 1986 Feb;126(2):259–265. doi: 10.1002/jcp.1041260216. [DOI] [PubMed] [Google Scholar]
- Koury M. J., Bondurant M. C., Rana S. S. Changes in erythroid membrane proteins during erythropoietin-mediated terminal differentiation. J Cell Physiol. 1987 Dec;133(3):438–448. doi: 10.1002/jcp.1041330304. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Woods C. Biogenesis of the red blood cell membrane-skeleton and the control of erythroid morphogenesis. Annu Rev Cell Biol. 1989;5:427–452. doi: 10.1146/annurev.cb.05.110189.002235. [DOI] [PubMed] [Google Scholar]
- Lehnert M. E., Lodish H. F. Unequal synthesis and differential degradation of alpha and beta spectrin during murine erythroid differentiation. J Cell Biol. 1988 Aug;107(2):413–426. doi: 10.1083/jcb.107.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehls V., Drenckhahn D., Joshi R., Bennett V. Adducin in erythrocyte precursor cells of rats and humans: expression and compartmentalization. Blood. 1991 Oct 1;78(7):1692–1696. [PubMed] [Google Scholar]
- Patel V. P., Lodish H. F. A fibronectin matrix is required for differentiation of murine erythroleukemia cells into reticulocytes. J Cell Biol. 1987 Dec;105(6 Pt 2):3105–3118. doi: 10.1083/jcb.105.6.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer S. R., Redman C. M. Biosynthesis of mouse erythrocyte membrane proteins by Friend erythroleukemia cells. Biochim Biophys Acta. 1981 Feb 20;641(1):254–263. doi: 10.1016/0005-2736(81)90589-7. [DOI] [PubMed] [Google Scholar]
- Sabban E. L., Sabatini D. D., Marchesi V. T., Adesnik M. Biosynthesis of erythrocyte membrane protein band 3 in DMSO-induced Friend erythroleukemia cells. J Cell Physiol. 1980 Aug;104(2):261–268. doi: 10.1002/jcp.1041040217. [DOI] [PubMed] [Google Scholar]
- Wagner S., Vogel R., Lietzke R., Koob R., Drenckhahn D. Immunochemical characterization of a band 3-like anion exchanger in collecting duct of human kidney. Am J Physiol. 1987 Aug;253(2 Pt 2):F213–F221. doi: 10.1152/ajprenal.1987.253.2.F213. [DOI] [PubMed] [Google Scholar]
- Woods C. M., Boyer B., Vogt P. K., Lazarides E. Control of erythroid differentiation: asynchronous expression of the anion transporter and the peripheral components of the membrane skeleton in AEV- and S13-transformed cells. J Cell Biol. 1986 Nov;103(5):1789–1798. doi: 10.1083/jcb.103.5.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]