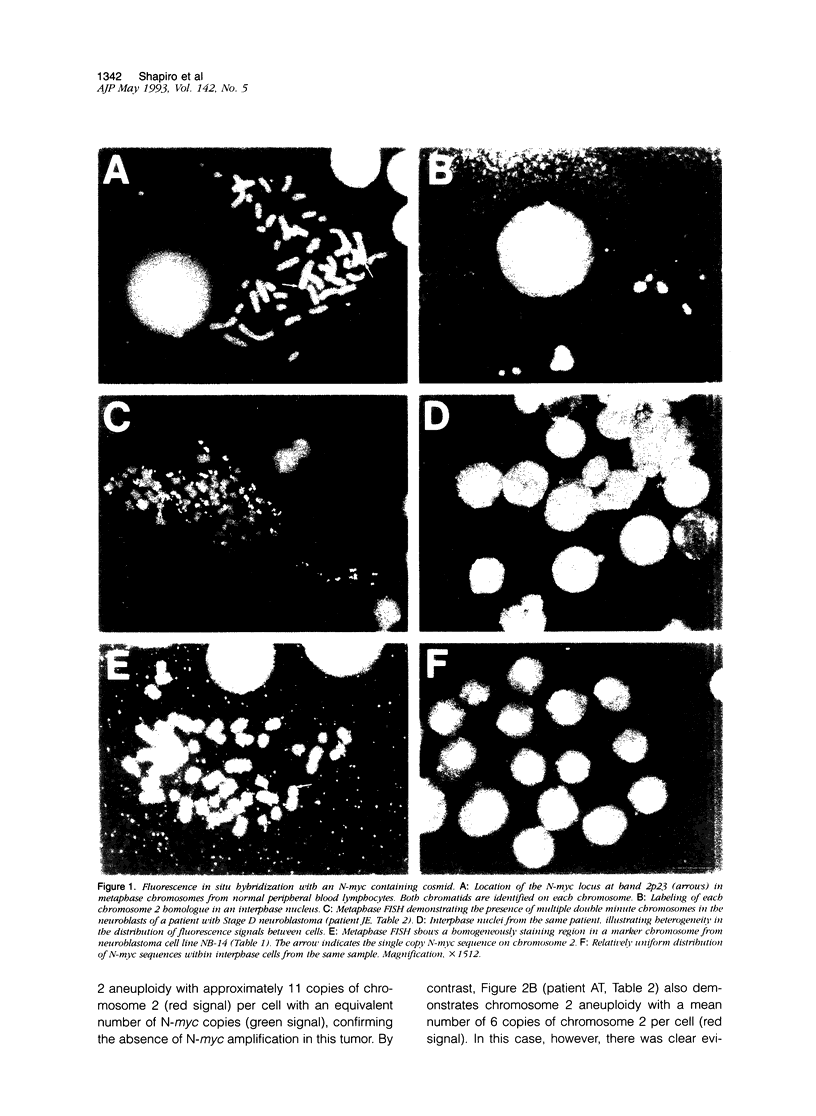
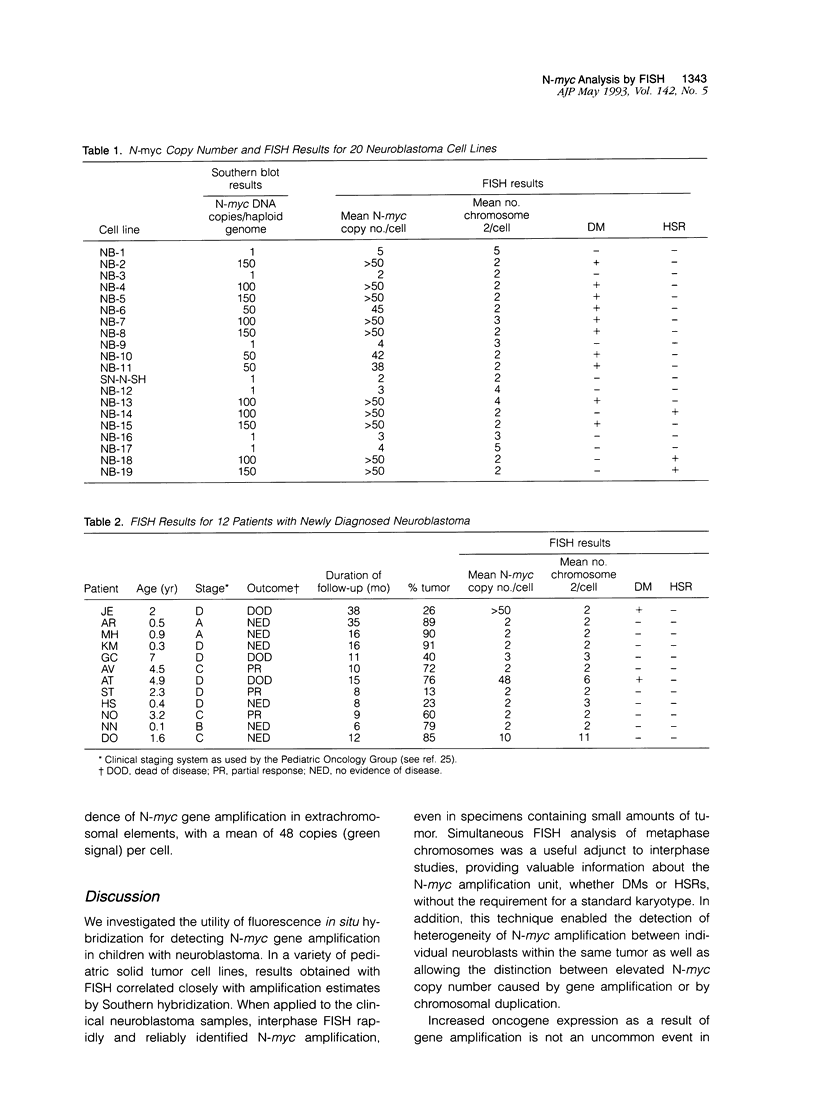
Abstract
We assessed fluorescence in situ hybridization (FISH) as an alternative to Southern blot analysis for determination of N-myc gene amplification in neuroblastoma. In the 44 pediatric solid tumor cell lines examined (20 neuroblastomas), the mean number of N-myc copies determined by FISH correlated closely with Southern blot results. There was wide intercellular variability in gene copy number in tumors that had evidence of amplification; however, tumors judged to be non-amplified completely lacked any cells with high N-myc copy number. FISH provided reliable estimates of N-myc amplification in 12 clinical samples even when the percentage of tumor was low. The other advantages of FISH over Southern blot analysis were speed and technical simplicity, ability to discern heterogeneous gene amplification among tumor cells in the same specimen, and capacity to determine the source of the amplified N-myc signal, whether extrachromosomal double-minute chromosomes, expanded intrachromosomal regions, or chromosome 2 aneuploidy. We conclude that FISH would refine the analysis of N-myc amplification in neuroblastoma and thus improve the assignment of patients to prognostic groups based on this unfavorable risk factor.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alex R., Sözeri O., Meyer S., Dildrop R. Determination of the DNA sequence recognized by the bHLH-zip domain of the N-Myc protein. Nucleic Acids Res. 1992 May 11;20(9):2257–2263. doi: 10.1093/nar/20.9.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodeur G. M., Green A. A., Hayes F. A., Williams K. J., Williams D. L., Tsiatis A. A. Cytogenetic features of human neuroblastomas and cell lines. Cancer Res. 1981 Nov;41(11 Pt 1):4678–4686. [PubMed] [Google Scholar]
- Brodeur G. M., Seeger R. C., Schwab M., Varmus H. E., Bishop J. M. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science. 1984 Jun 8;224(4653):1121–1124. doi: 10.1126/science.6719137. [DOI] [PubMed] [Google Scholar]
- Cohen P. S., Seeger R. C., Triche T. J., Israel M. A. Detection of N-myc gene expression in neuroblastoma tumors by in situ hybridization. Am J Pathol. 1988 Jun;131(3):391–397. [PMC free article] [PubMed] [Google Scholar]
- Dias P., Kumar P., Marsden H. B., Gattamaneni H. R., Heighway J., Kumar S. N-myc gene is amplified in alveolar rhabdomyosarcomas (RMS) but not in embryonal RMS. Int J Cancer. 1990 Apr 15;45(4):593–596. doi: 10.1002/ijc.2910450403. [DOI] [PubMed] [Google Scholar]
- Garson J. A., Clayton J., McIntyre P., Kemshead J. T. N-myc oncogene amplification in rhabdomyosarcoma at release. Lancet. 1986 Jun 28;1(8496):1496–1496. doi: 10.1016/s0140-6736(86)91527-8. [DOI] [PubMed] [Google Scholar]
- Grady-Leopardi E. F., Schwab M., Ablin A. R., Rosenau W. Detection of N-myc oncogene expression in human neuroblastoma by in situ hybridization and blot analysis: relationship to clinical outcome. Cancer Res. 1986 Jun;46(6):3196–3199. [PubMed] [Google Scholar]
- Hayashi Y., Kanda N., Inaba T., Hanada R., Nagahara N., Muchi H., Yamamoto K. Cytogenetic findings and prognosis in neuroblastoma with emphasis on marker chromosome 1. Cancer. 1989 Jan 1;63(1):126–132. doi: 10.1002/1097-0142(19890101)63:1<126::aid-cncr2820630120>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Johnson B. E., Ihde D. C., Makuch R. W., Gazdar A. F., Carney D. N., Oie H., Russell E., Nau M. M., Minna J. D. myc family oncogene amplification in tumor cell lines established from small cell lung cancer patients and its relationship to clinical status and course. J Clin Invest. 1987 Jun;79(6):1629–1634. doi: 10.1172/JCI112999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallioniemi O. P., Kallioniemi A., Kurisu W., Thor A., Chen L. C., Smith H. S., Waldman F. M., Pinkel D., Gray J. W. ERBB2 amplification in breast cancer analyzed by fluorescence in situ hybridization. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5321–5325. doi: 10.1073/pnas.89.12.5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko Y., Kanda N., Maseki N., Sakurai M., Tsuchida Y., Takeda T., Okabe I., Sakurai M. Different karyotypic patterns in early and advanced stage neuroblastomas. Cancer Res. 1987 Jan 1;47(1):311–318. [PubMed] [Google Scholar]
- Kinzler K. W., Bigner S. H., Bigner D. D., Trent J. M., Law M. L., O'Brien S. J., Wong A. J., Vogelstein B. Identification of an amplified, highly expressed gene in a human glioma. Science. 1987 Apr 3;236(4797):70–73. doi: 10.1126/science.3563490. [DOI] [PubMed] [Google Scholar]
- Lawrence J. B., Villnave C. A., Singer R. H. Sensitive, high-resolution chromatin and chromosome mapping in situ: presence and orientation of two closely integrated copies of EBV in a lymphoma line. Cell. 1988 Jan 15;52(1):51–61. doi: 10.1016/0092-8674(88)90530-2. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Murphree A. L., Benedict W. F. Expression and amplification of the N-myc gene in primary retinoblastoma. 1984 May 31-Jun 6Nature. 309(5967):458–460. doi: 10.1038/309458a0. [DOI] [PubMed] [Google Scholar]
- Look A. T., Hayes F. A., Shuster J. J., Douglass E. C., Castleberry R. P., Bowman L. C., Smith E. I., Brodeur G. M. Clinical relevance of tumor cell ploidy and N-myc gene amplification in childhood neuroblastoma: a Pediatric Oncology Group study. J Clin Oncol. 1991 Apr;9(4):581–591. doi: 10.1200/JCO.1991.9.4.581. [DOI] [PubMed] [Google Scholar]
- Morris S. W., Valentine M. B., Shapiro D. N., Sublett J. E., Deaven L. L., Foust J. T., Roberts W. M., Cerretti D. P., Look A. T. Reassignment of the human CSF1 gene to chromosome 1p13-p21. Blood. 1991 Oct 15;78(8):2013–2020. [PubMed] [Google Scholar]
- Rudduck C., Lukeis R. E., McRobert T. L., Chow C. W., Garson O. M. Chromosomal localization of amplified N-myc in neuroblastoma cells using a biotinylated probe. Cancer Genet Cytogenet. 1992 Jan;58(1):55–59. doi: 10.1016/0165-4608(92)90134-t. [DOI] [PubMed] [Google Scholar]
- Schwab M., Alitalo K., Klempnauer K. H., Varmus H. E., Bishop J. M., Gilbert F., Brodeur G., Goldstein M., Trent J. Amplified DNA with limited homology to myc cellular oncogene is shared by human neuroblastoma cell lines and a neuroblastoma tumour. Nature. 1983 Sep 15;305(5931):245–248. doi: 10.1038/305245a0. [DOI] [PubMed] [Google Scholar]
- Schwab M., Varmus H. E., Bishop J. M., Grzeschik K. H., Naylor S. L., Sakaguchi A. Y., Brodeur G., Trent J. Chromosome localization in normal human cells and neuroblastomas of a gene related to c-myc. Nature. 1984 Mar 15;308(5956):288–291. doi: 10.1038/308288a0. [DOI] [PubMed] [Google Scholar]
- Seeger R. C., Brodeur G. M., Sather H., Dalton A., Siegel S. E., Wong K. Y., Hammond D. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N Engl J Med. 1985 Oct 31;313(18):1111–1116. doi: 10.1056/NEJM198510313131802. [DOI] [PubMed] [Google Scholar]
- Slamon D. J., Clark G. M., Wong S. G., Levin W. J., Ullrich A., McGuire W. L. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987 Jan 9;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- Slamon D. J., deKernion J. B., Verma I. M., Cline M. J. Expression of cellular oncogenes in human malignancies. Science. 1984 Apr 20;224(4646):256–262. doi: 10.1126/science.6538699. [DOI] [PubMed] [Google Scholar]
- Wahl G. M. The importance of circular DNA in mammalian gene amplification. Cancer Res. 1989 Mar 15;49(6):1333–1340. [PubMed] [Google Scholar]