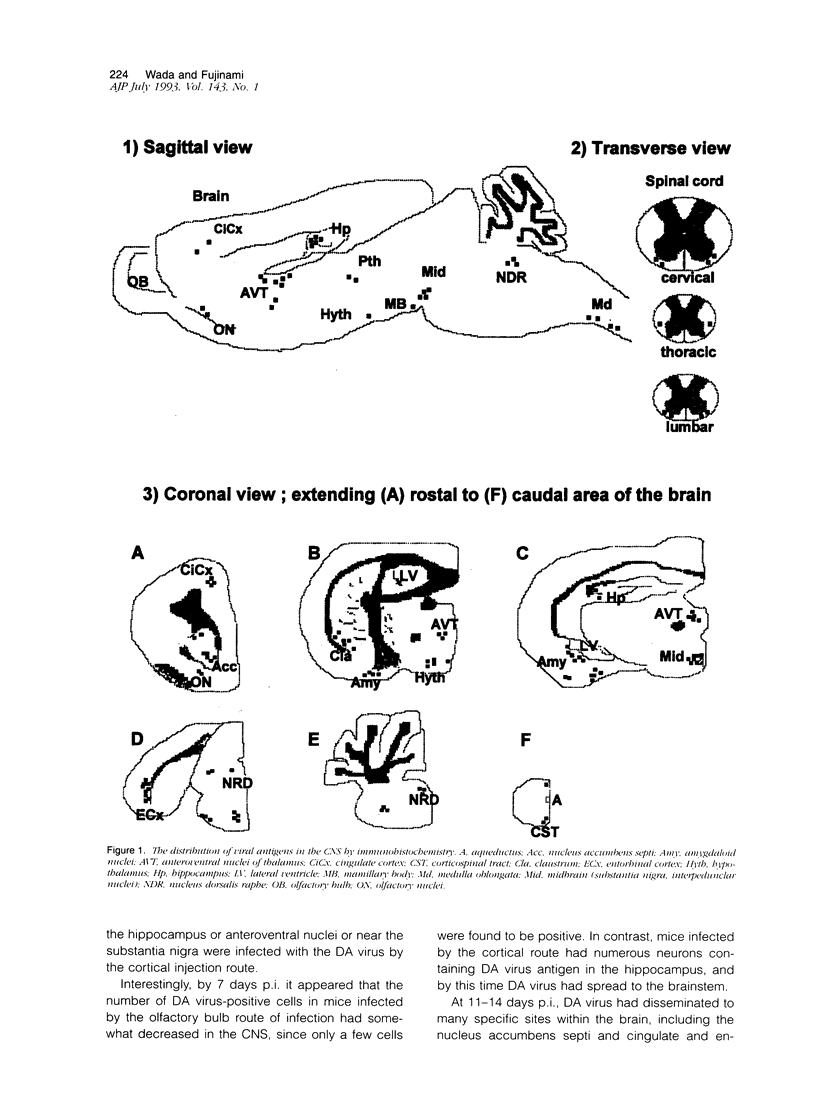
Abstract
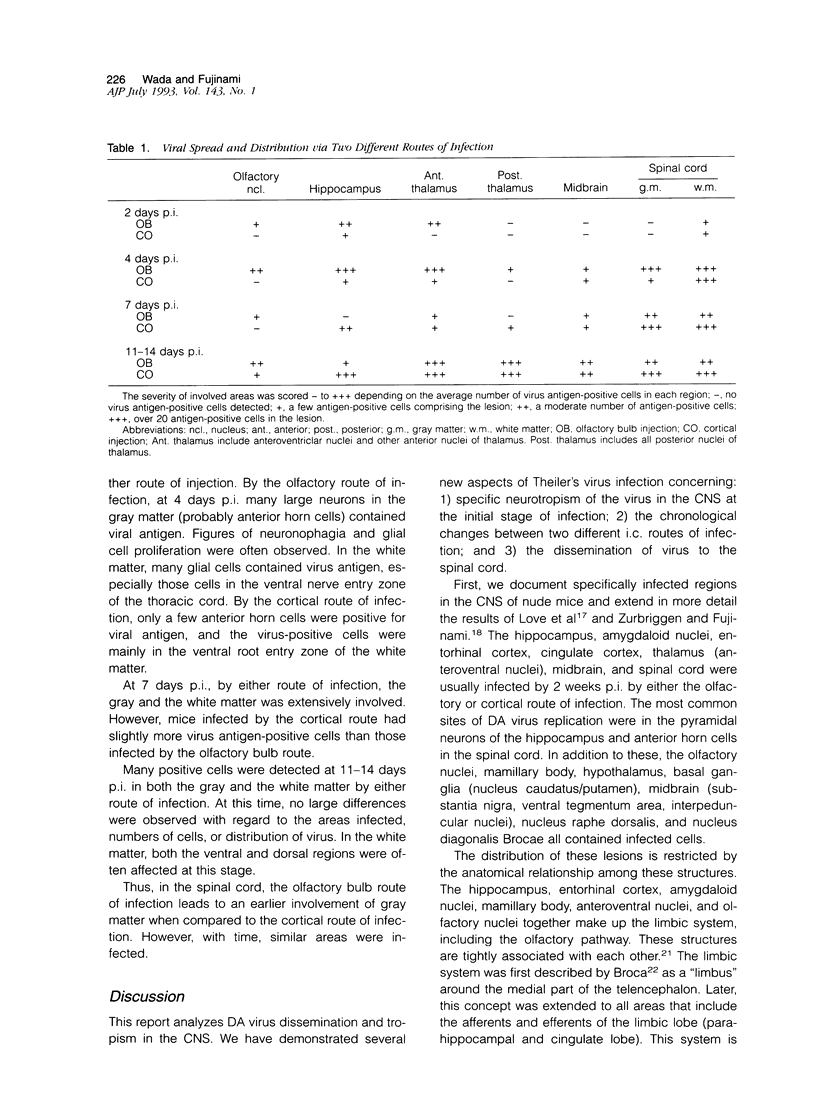
Theiler's murine encephalomyelitis virus (TMEV) infection of mice can produce a biphasic disease of the central nervous system (CNS). Most susceptible strains of mice survive the acute infection and develop a chronic demyelinating disease. In this report, we analyzed the routes of spread of TMEV within the CNS of nude mice and target sites eventually infected in the CNS. Compared to the immunocompetent mouse, in which an antiviral immune response is mounted but virus persists, the nude mouse develops a severe encephalomyelitis due to the lack of functional T lymphocytes and provides a useful model for the study of viral dissemination. We demonstrated, by immunohistochemistry, the presence of viral antigen in defined regions of the CNS, corresponding to various structures of the limbic system. In addition, we found a different time course for viral spread using two different sites of intracerebral inoculation, ie, via the olfactory bulb or the cortex. Limbic structures were rapidly infected following olfactory bulb infection and then showed a decrease in viral load, presumably due to loss of target neurons. Using either route of infection, the virus was able to disseminate to similar regions. These results indicate that limbic structures and their connections are very important for the spread of TMEV in the brain. In the spinal cord, not only neuronal but hematogenous pathways were suspected to be involved in the dissemination of Theiler's virus.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. R., Field H. J. The distribution of herpes simplex type 1 antigen in mouse central nervous system after different routes of inoculation. J Neurol Sci. 1983 Aug;60(2):181–195. doi: 10.1016/0022-510x(83)90061-8. [DOI] [PubMed] [Google Scholar]
- Borrow P., Nash A. A. Susceptibility to Theiler's virus-induced demyelinating disease correlates with astrocyte class II induction and antigen presentation. Immunology. 1992 May;76(1):133–139. [PMC free article] [PubMed] [Google Scholar]
- Borrow P., Tonks P., Welsh C. J., Nash A. A. The role of CD8+T cells in the acute and chronic phases of Theiler's murine encephalomyelitis virus-induced disease in mice. J Gen Virol. 1992 Jul;73(Pt 7):1861–1865. doi: 10.1099/0022-1317-73-7-1861. [DOI] [PubMed] [Google Scholar]
- Brahic M., Stroop W. G., Baringer J. R. Theiler's virus persists in glial cells during demyelinating disease. Cell. 1981 Oct;26(1 Pt 1):123–128. doi: 10.1016/0092-8674(81)90040-4. [DOI] [PubMed] [Google Scholar]
- Clatch R. J., Lipton H. L., Miller S. D. Characterization of Theiler's murine encephalomyelitis virus (TMEV)-specific delayed-type hypersensitivity responses in TMEV-induced demyelinating disease: correlation with clinical signs. J Immunol. 1986 Feb 1;136(3):920–927. [PubMed] [Google Scholar]
- DANIELS J. B., PAPPENHEIMER A. M., RICHARDSON S. Observations on encephalomyelitis of mice (DA strain). J Exp Med. 1952 Dec;96(6):517–530. doi: 10.1084/jem.96.6.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Canto M. C., Lipton H. L. Primary demyelination in Theiler's virus infection. An ultrastructural study. Lab Invest. 1975 Dec;33(6):626–637. [PubMed] [Google Scholar]
- Juhler M., Blasberg R. G., Fenstermacher J. D., Patlak C. S., Paulson O. B. A spatial analysis of the blood-brain barrier damage in experimental allergic encephalomyelitis. J Cereb Blood Flow Metab. 1985 Dec;5(4):545–553. doi: 10.1038/jcbfm.1985.82. [DOI] [PubMed] [Google Scholar]
- Lindsley M. D., Rodriguez M. Characterization of the inflammatory response in the central nervous system of mice susceptible or resistant to demyelination by Theiler's virus. J Immunol. 1989 Apr 15;142(8):2677–2682. [PubMed] [Google Scholar]
- Lipton H. L., Canto C. D. Contrasting effects of immunosuppression on Theiler's virus infection in mice. Infect Immun. 1977 Mar;15(3):903–909. doi: 10.1128/iai.15.3.903-909.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton H. L., Dal Canto M. C. Theiler's virus-induced demyelination: prevention by immunosuppression. Science. 1976 Apr 2;192(4234):62–64. doi: 10.1126/science.176726. [DOI] [PubMed] [Google Scholar]
- Lipton H. L. Theiler's virus infection in mice: an unusual biphasic disease process leading to demyelination. Infect Immun. 1975 May;11(5):1147–1155. doi: 10.1128/iai.11.5.1147-1155.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love S. Distribution of Theiler's virus in the CNS of athymic nude mice: effect of varying the route of inoculation. J Neurol Sci. 1987 Oct;81(1):55–66. doi: 10.1016/0022-510x(87)90183-3. [DOI] [PubMed] [Google Scholar]
- Martin X., Dolivo M. Neuronal and transneuronal tracing in the trigeminal system of the rat using the herpes virus suis. Brain Res. 1983 Aug 29;273(2):253–276. doi: 10.1016/0006-8993(83)90850-8. [DOI] [PubMed] [Google Scholar]
- McLean J. H., Shipley M. T. Serotonergic afferents to the rat olfactory bulb: I. Origins and laminar specificity of serotonergic inputs in the adult rat. J Neurosci. 1987 Oct;7(10):3016–3028. doi: 10.1523/JNEUROSCI.07-10-03016.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. D., Clatch R. J., Pevear D. C., Trotter J. L., Lipton H. L. Class II-restricted T cell responses in Theiler's murine encephalomyelitis virus (TMEV)-induced demyelinating disease. I. Cross-specificity among TMEV substrains and related picornaviruses, but not myelin proteins. J Immunol. 1987 Jun 1;138(11):3776–3784. [PubMed] [Google Scholar]
- Reinacher M., Bonin J., Narayan O., Scholtissek C. Pathogenesis of neurovirulent influenza A virus infection in mice. Route of entry of virus into brain determines infection of different populations of cells. Lab Invest. 1983 Dec;49(6):686–692. [PubMed] [Google Scholar]
- Rodriguez M., Leibowitz J. L., Lampert P. W. Persistent infection of oligodendrocytes in Theiler's virus-induced encephalomyelitis. Ann Neurol. 1983 Apr;13(4):426–433. doi: 10.1002/ana.410130409. [DOI] [PubMed] [Google Scholar]
- Rodriguez M., Oleszak E., Leibowitz J. Theiler's murine encephalomyelitis: a model of demyelination and persistence of virus. Crit Rev Immunol. 1987;7(4):325–365. [PubMed] [Google Scholar]
- Roos R. P., Wollmann R. DA strain of Theiler's murine encephalomyelitis virus induces demyelination in nude mice. Ann Neurol. 1984 May;15(5):494–499. doi: 10.1002/ana.410150516. [DOI] [PubMed] [Google Scholar]
- Rosenthal A., Fujinami R. S., Lampert P. W. Mechanism of Theiler's virus-induced demyelination in nude mice. Lab Invest. 1986 May;54(5):515–522. [PubMed] [Google Scholar]
- Tyler K. L., McPhee D. A., Fields B. N. Distinct pathways of viral spread in the host determined by reovirus S1 gene segment. Science. 1986 Aug 15;233(4765):770–774. doi: 10.1126/science.3016895. [DOI] [PubMed] [Google Scholar]
- Yamada M., Zurbriggen A., Fujinami R. S. Monoclonal antibody to Theiler's murine encephalomyelitis virus defines a determinant on myelin and oligodendrocytes, and augments demyelination in experimental allergic encephalomyelitis. J Exp Med. 1990 Jun 1;171(6):1893–1907. doi: 10.1084/jem.171.6.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M., Zurbriggen A., Fujinami R. S. The relationship between viral RNA, myelin-specific mRNAs, and demyelination in central nervous system disease during Theiler's virus infection. Am J Pathol. 1990 Dec;137(6):1467–1479. [PMC free article] [PubMed] [Google Scholar]
- Zurbriggen A., Fujinami R. S. Theiler's virus infection in nude mice: viral RNA in vascular endothelial cells. J Virol. 1988 Oct;62(10):3589–3596. doi: 10.1128/jvi.62.10.3589-3596.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Záborszky L., Carlsen J., Brashear H. R., Heimer L. Cholinergic and GABAergic afferents to the olfactory bulb in the rat with special emphasis on the projection neurons in the nucleus of the horizontal limb of the diagonal band. J Comp Neurol. 1986 Jan 22;243(4):488–509. doi: 10.1002/cne.902430405. [DOI] [PubMed] [Google Scholar]