Abstract
The lacrimal gland (LG) immunopathology of Sjögren's syndrome (SS) consists of a proliferation of B and CD4 lymphocytes surrounding epithelial structures (Pepose JS, et al: Ophthalmology 1990, 97:1599-1605). Based on the detection of EBV genomes in a greater percentage of SS than normal LG biopsies, we previously postulated that Epstein-Barr virus (EBV) is a risk factor for LG lymphoproliferation in SS (Pflugfelder SC, et al: Ophthalmology 1990, 97:976-984). The purpose of this study was to determine the cellular site(s) of infection, virus type, and antigen expression of EBV infecting normal and SS LGs. EBV DNA was detected by in situ hybridization in intraductal epithelia in 13-33% of lobules in 21% of normal LGs and in cells in areas of B lymphoproliferation as well as the majority of epithelia in 86% of SS LGs. EBV genomic sequences were amplified from 36% of normal and 88% of SS LG biopsies by polymerase chain reaction. Only type 1 EBV sequences were amplified in SS LGs; in contrast EBV nuclear antigen 2-deleted but not type 1 sequences were amplified in normal LGs. Immunohistochemistry with EBV-specific monoclonal antibodies was performed on normal and SS LGs. No EBV antigens were detected in normal LGs. In contrast, latent antigens (latent membrane protein, EBV nuclear antigen 2) were detected in lymphocytes in areas of B lymphoproliferation, and early and late lytic cycle antigens were observed in epithelia in SS LGs. These studies suggest that EBV may play a role in the LG B lymphoproliferation and epithelial pathologic changes observed in SS.
Full text
PDF

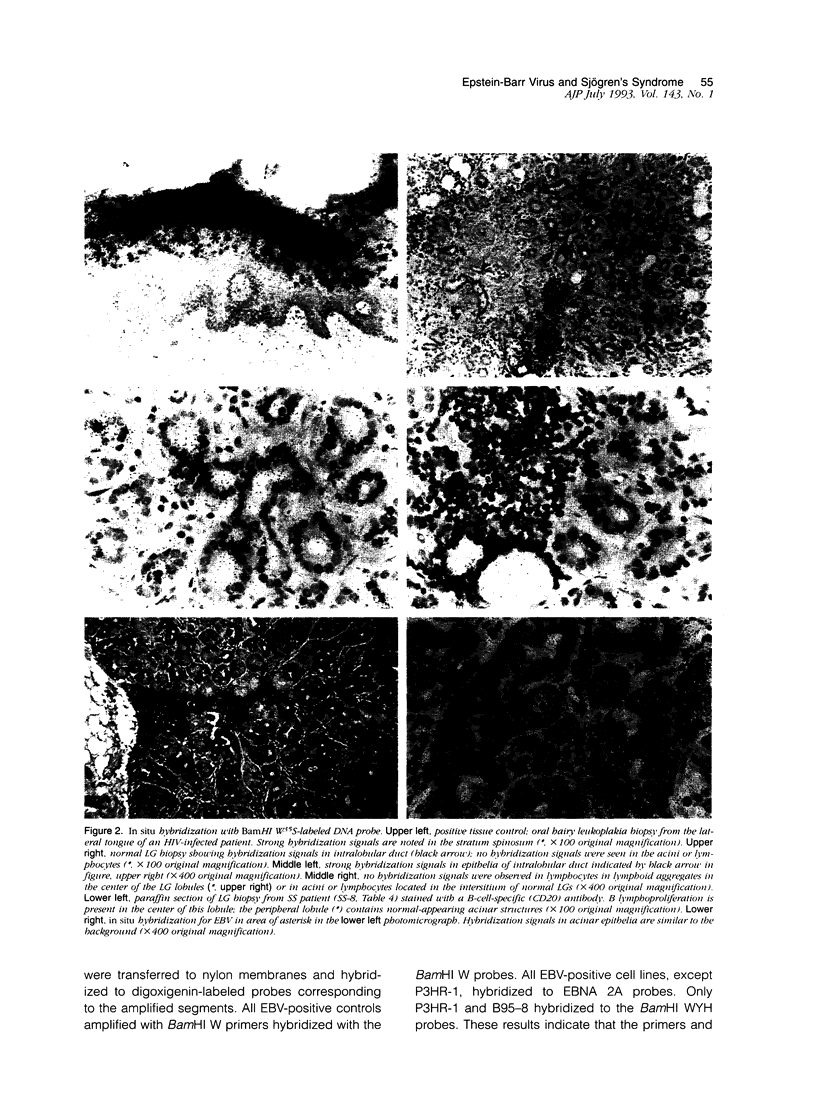
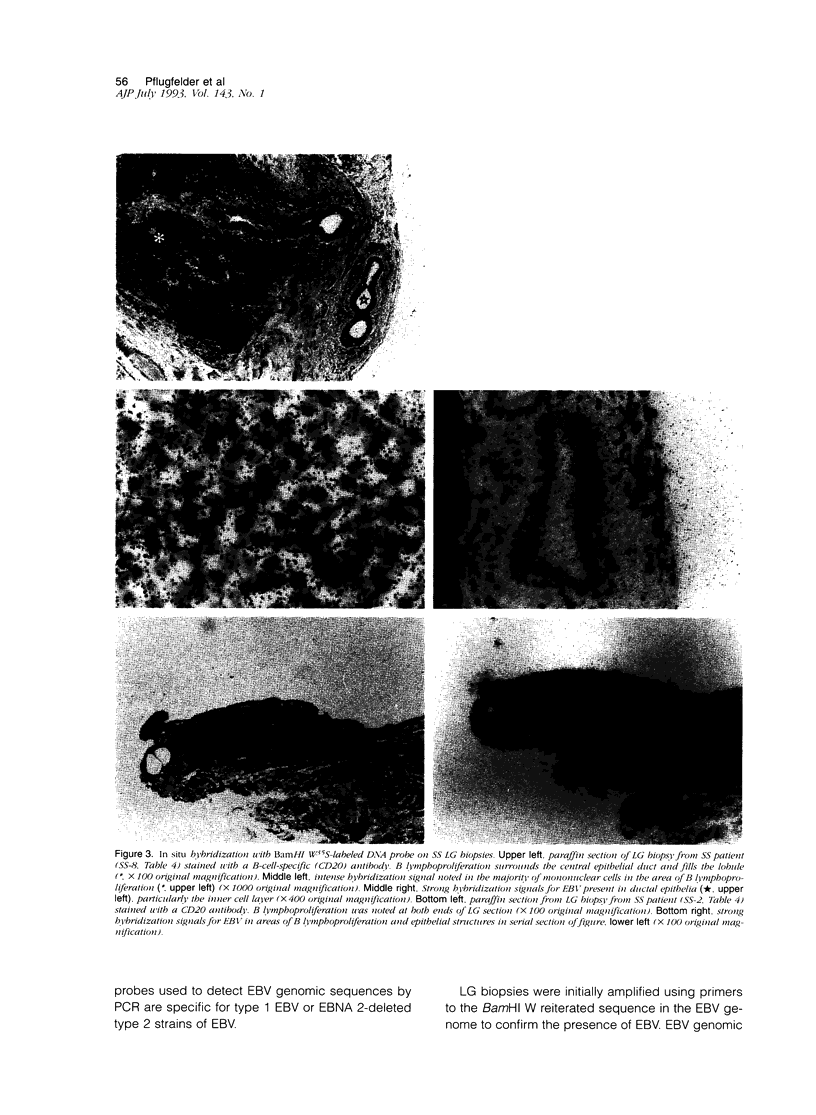
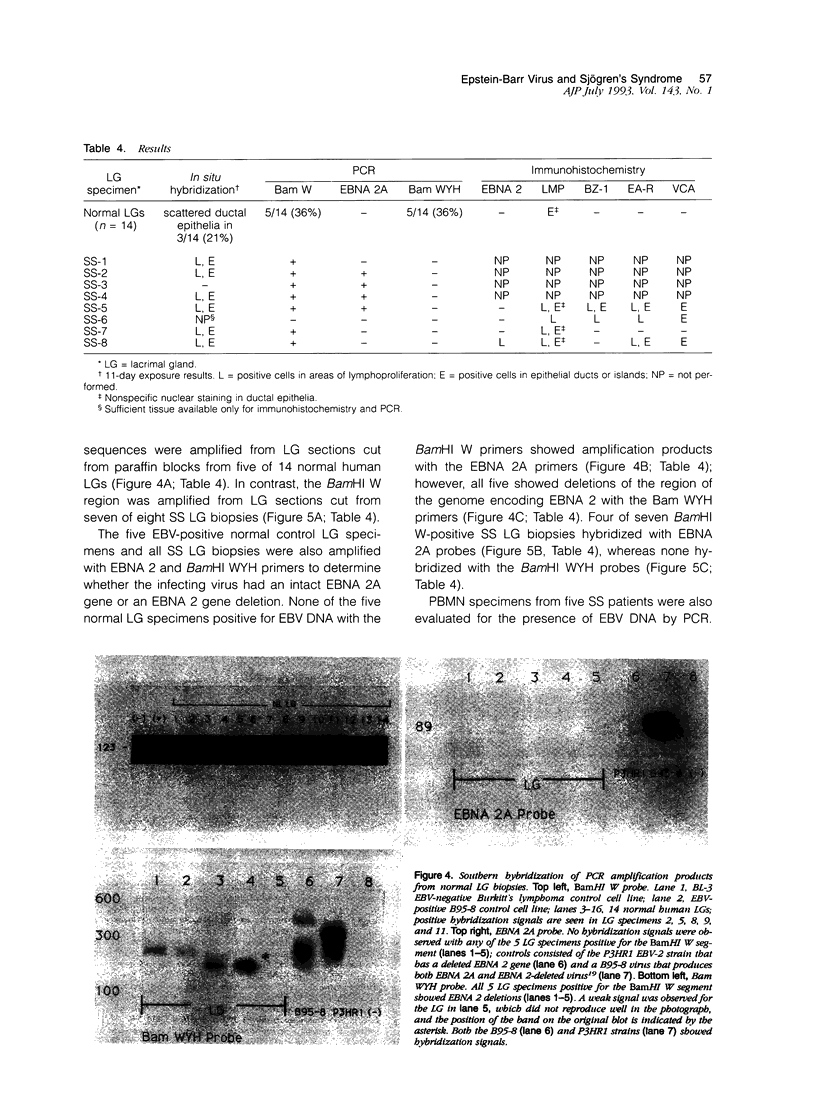
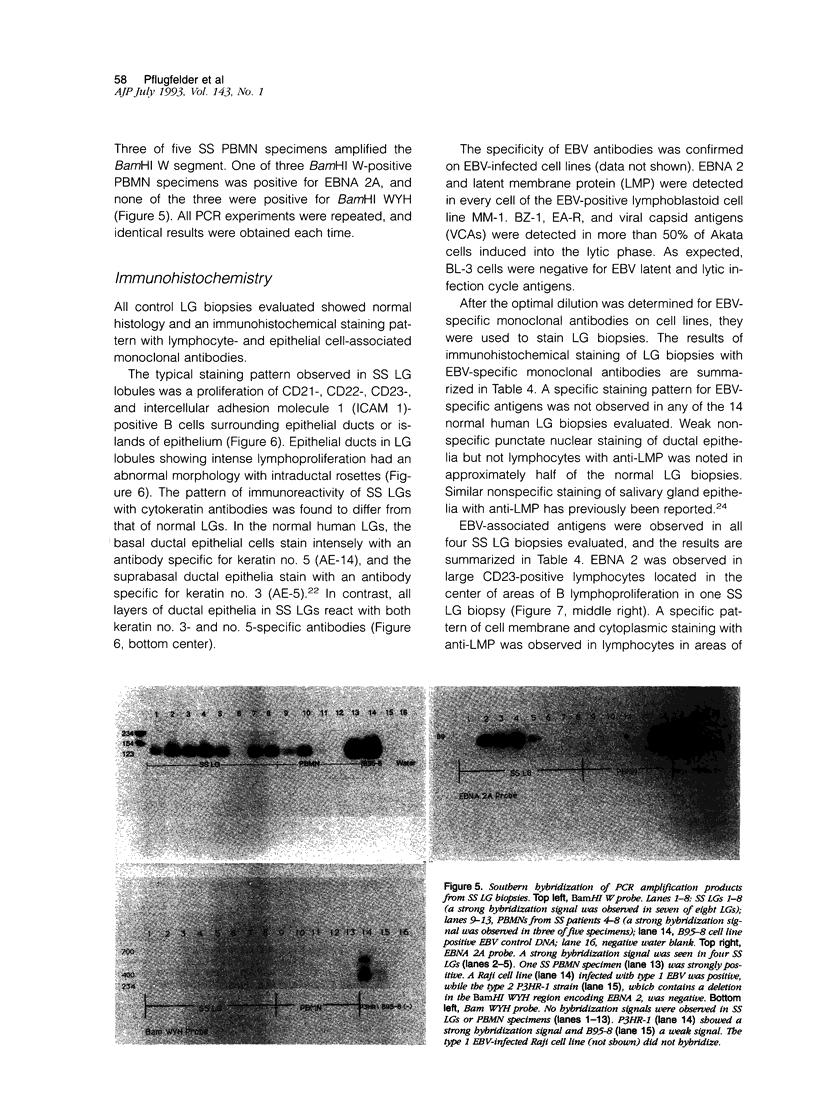
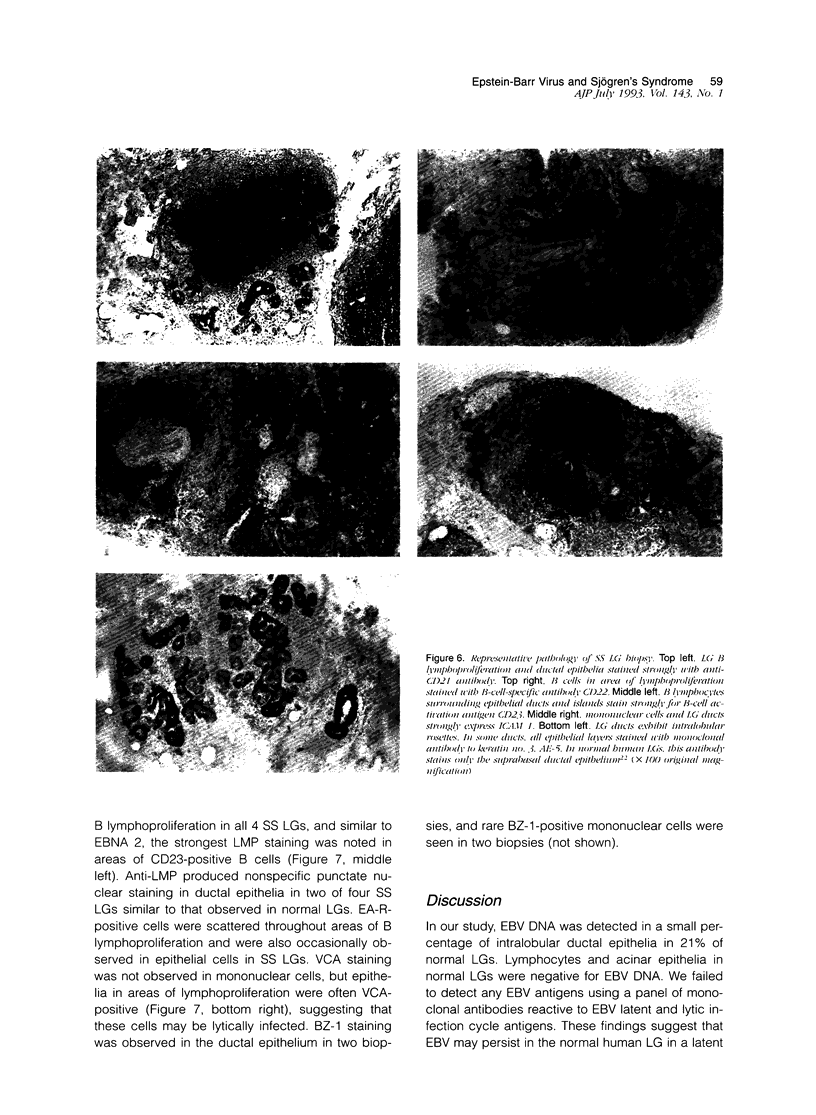
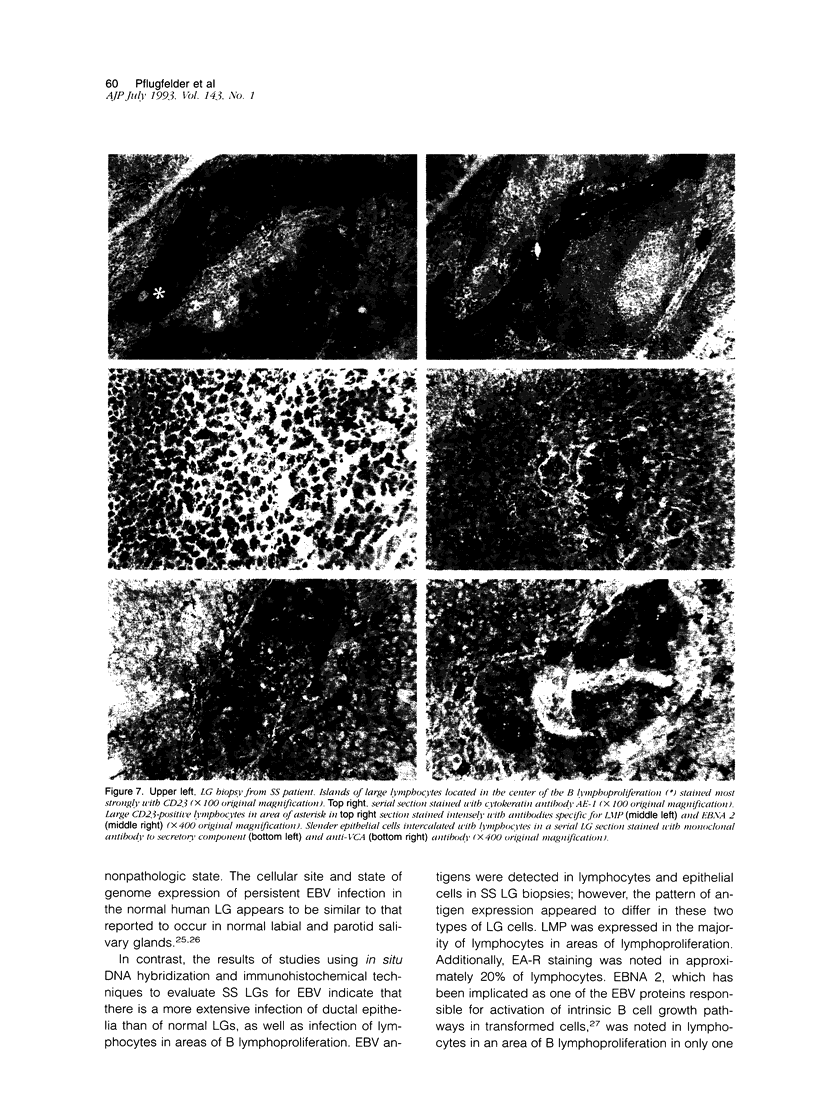




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson T. C., 3rd, Fox R. I., Frisman D. M., Howell F. V. Immunohistologic analysis of lymphoid infiltrates in primary Sjogren's syndrome using monoclonal antibodies. J Immunol. 1983 Jan;130(1):203–208. [PubMed] [Google Scholar]
- Arnett F. C., Bias W. B., Reveille J. D. Genetic studies in Sjögren's syndrome and systemic lupus erythematosus. J Autoimmun. 1989 Aug;2(4):403–413. doi: 10.1016/0896-8411(89)90169-8. [DOI] [PubMed] [Google Scholar]
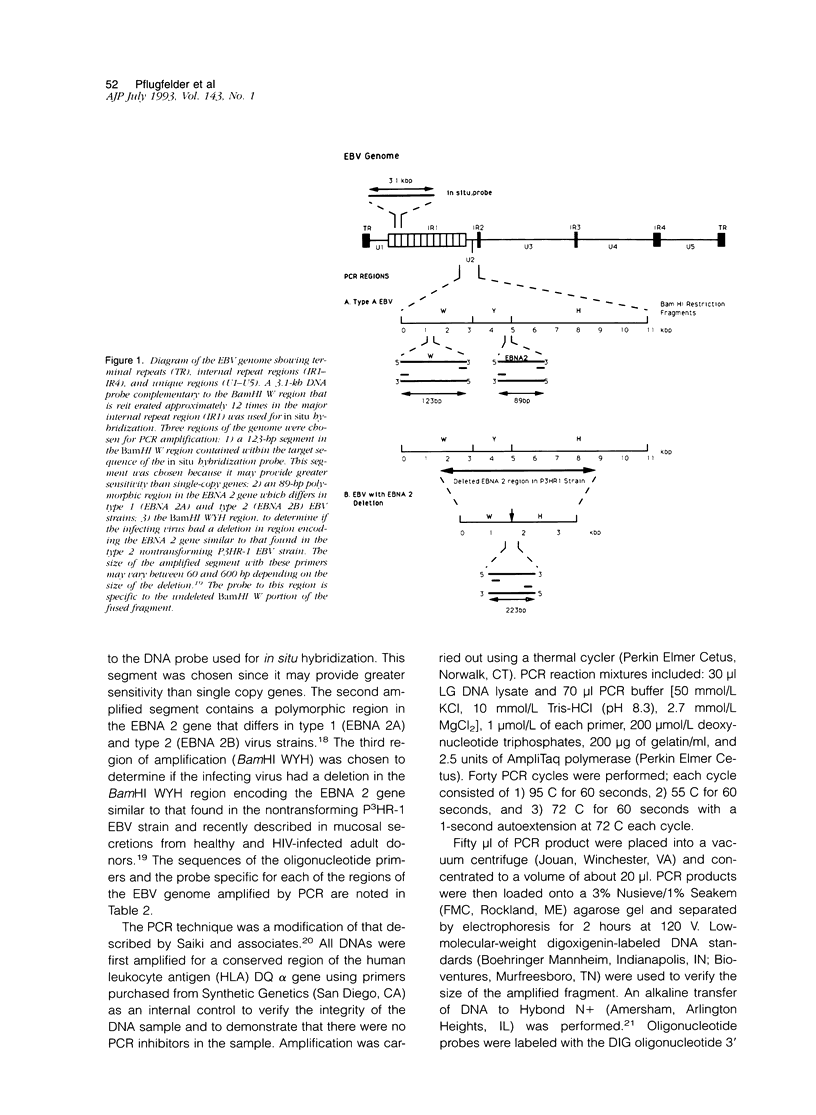
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Bornkamm G. W., Hudewentz J., Freese U. K., Zimber U. Deletion of the nontransforming Epstein-Barr virus strain P3HR-1 causes fusion of the large internal repeat to the DSL region. J Virol. 1982 Sep;43(3):952–968. doi: 10.1128/jvi.43.3.952-968.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandsma J., Miller G. Nucleic acid spot hybridization: rapid quantitative screening of lymphoid cell lines for Epstein-Barr viral DNA. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6851–6855. doi: 10.1073/pnas.77.11.6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. I., Wang F., Kieff E. Epstein-Barr virus nuclear protein 2 mutations define essential domains for transformation and transactivation. J Virol. 1991 May;65(5):2545–2554. doi: 10.1128/jvi.65.5.2545-2554.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daibata M., Humphreys R. E., Takada K., Sairenji T. Activation of latent EBV via anti-IgG-triggered, second messenger pathways in the Burkitt's lymphoma cell line Akata. J Immunol. 1990 Jun 15;144(12):4788–4793. [PubMed] [Google Scholar]
- Deacon E. M., Matthews J. B., Potts A. J., Hamburger J., Bevan I. S., Young L. S. Detection of Epstein-Barr virus antigens and DNA in major and minor salivary glands using immunocytochemistry and polymerase chain reaction: possible relationship with Sjogren's syndrome. J Pathol. 1991 Apr;163(4):351–360. doi: 10.1002/path.1711630413. [DOI] [PubMed] [Google Scholar]
- Font R. L., Yanoff M., Zimmerman L. E. Benign lymphoepithelial lesion of the lacrimal gland and its relationship to Sjögren's syndrome. Am J Clin Pathol. 1967 Oct;48(4):365–376. doi: 10.1093/ajcp/48.4.365. [DOI] [PubMed] [Google Scholar]
- Fox R. I., Carstens S. A., Fong S., Robinson C. A., Howell F., Vaughan J. H. Use of monoclonal antibodies to analyze peripheral blood and salivary gland lymphocyte subsets in Sjögren's syndrome. Arthritis Rheum. 1982 Apr;25(4):419–426. doi: 10.1002/art.1780250410. [DOI] [PubMed] [Google Scholar]
- Fox R. I., Pearson G., Vaughan J. H. Detection of Epstein-Barr virus-associated antigens and DNA in salivary gland biopsies from patients with Sjogren's syndrome. J Immunol. 1986 Nov 15;137(10):3162–3168. [PubMed] [Google Scholar]
- Fox R. I., Robinson C. A., Curd J. G., Kozin F., Howell F. V. Sjögren's syndrome. Proposed criteria for classification. Arthritis Rheum. 1986 May;29(5):577–585. doi: 10.1002/art.1780290501. [DOI] [PubMed] [Google Scholar]
- Gaston J. S., Rowe M., Bacon P. Sjögren's syndrome after infection by Epstein-Barr virus. J Rheumatol. 1990 Apr;17(4):558–561. [PubMed] [Google Scholar]
- Kassan S. S., Thomas T. L., Moutsopoulos H. M., Hoover R., Kimberly R. P., Budman D. R., Costa J., Decker J. L., Chused T. M. Increased risk of lymphoma in sicca syndrome. Ann Intern Med. 1978 Dec;89(6):888–892. doi: 10.7326/0003-4819-89-6-888. [DOI] [PubMed] [Google Scholar]
- Lawrence J. B., Villnave C. A., Singer R. H. Sensitive, high-resolution chromatin and chromosome mapping in situ: presence and orientation of two closely integrated copies of EBV in a lymphoma line. Cell. 1988 Jan 15;52(1):51–61. doi: 10.1016/0092-8674(88)90530-2. [DOI] [PubMed] [Google Scholar]
- Lenoir G. M., Preud'homme J. L., Bernheim A., Berger R. Correlation between immunoglobulin light chain expression and variant translocation in Burkitt's lymphoma. Nature. 1982 Jul 29;298(5873):474–476. doi: 10.1038/298474a0. [DOI] [PubMed] [Google Scholar]
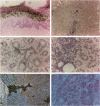
- Mariette X., Gozlan J., Clerc D., Bisson M., Morinet F. Detection of Epstein-Barr virus DNA by in situ hybridization and polymerase chain reaction in salivary gland biopsy specimens from patients with Sjögren's syndrome. Am J Med. 1991 Mar;90(3):286–294. [PubMed] [Google Scholar]
- Miller G., Coope D., Niederman J., Pagano J. Biological properties and viral surface antigens of Burkitt lymphoma- and mononucleosis- derived strains of Epstein-Barr virus released from transformed marmoset cells. J Virol. 1976 Jun;18(3):1071–1080. doi: 10.1128/jvi.18.3.1071-1080.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misko I. S., Schmidt C., Honeyman M., Soszynski T. D., Sculley T. B., Burrows S. R., Moss D. J., Burman K. Failure of Epstein-Barr virus-specific cytotoxic T lymphocytes to lyse B cells transformed with the B95-8 strain is mapped to an epitope that associates with the HLA-B8 antigen. Clin Exp Immunol. 1992 Jan;87(1):65–70. doi: 10.1111/j.1365-2249.1992.tb06414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M. D., Cannon M. J., Sewall A., Finlayson M., Okimoto M., Nemerow G. R. Inhibition of Epstein-Barr virus infection in vitro and in vivo by soluble CR2 (CD21) containing two short consensus repeats. J Virol. 1991 Jul;65(7):3559–3565. doi: 10.1128/jvi.65.7.3559-3565.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss D. J., Misko I. S., Burrows S. R., Burman K., McCarthy R., Sculley T. B. Cytotoxic T-cell clones discriminate between A- and B-type Epstein-Barr virus transformants. Nature. 1988 Feb 25;331(6158):719–721. doi: 10.1038/331719a0. [DOI] [PubMed] [Google Scholar]
- Murray R. J., Young L. S., Calender A., Gregory C. D., Rowe M., Lenoir G. M., Rickinson A. B. Different patterns of Epstein-Barr virus gene expression and of cytotoxic T-cell recognition in B-cell lines infected with transforming (B95.8) or nontransforming (P3HR1) virus strains. J Virol. 1988 Mar;62(3):894–901. doi: 10.1128/jvi.62.3.894-901.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngan B. Y., Warnke R. A., Wilson M., Takagi K., Cleary M. L., Dorfman R. F. Monocytoid B-cell lymphoma: a study of 36 cases. Hum Pathol. 1991 May;22(5):409–421. doi: 10.1016/0046-8177(91)90125-9. [DOI] [PubMed] [Google Scholar]
- Pepose J. S., Akata R. F., Pflugfelder S. C., Voigt W. Mononuclear cell phenotypes and immunoglobulin gene rearrangements in lacrimal gland biopsies from patients with Sjögren's syndrome. Ophthalmology. 1990 Dec;97(12):1599–1605. doi: 10.1016/s0161-6420(90)32372-2. [DOI] [PubMed] [Google Scholar]
- Pflugfelder S. C., Crouse C. A., Atherton S. S. Ophthalmic manifestations of Epstein-Barr virus infection. Int Ophthalmol Clin. 1993 Winter;33(1):95–101. doi: 10.1097/00004397-199303310-00009. [DOI] [PubMed] [Google Scholar]
- Pflugfelder S. C., Crouse C., Pereira I., Atherton S. Amplification of Epstein-Barr virus genomic sequences in blood cells, lacrimal glands, and tears from primary Sjögren's syndrome patients. Ophthalmology. 1990 Aug;97(8):976–984. doi: 10.1016/s0161-6420(90)32476-4. [DOI] [PubMed] [Google Scholar]
- Pflugfelder S. C., Roussel T. J., Culbertson W. W. Primary Sjögren's syndrome after infectious mononucleosis. JAMA. 1987 Feb 27;257(8):1049–1050. [PubMed] [Google Scholar]
- Pflugfelder S. C., Tseng S. C., Pepose J. S., Fletcher M. A., Klimas N., Feuer W. Epstein-Barr virus infection and immunologic dysfunction in patients with aqueous tear deficiency. Ophthalmology. 1990 Mar;97(3):313–323. doi: 10.1016/s0161-6420(90)32595-2. [DOI] [PubMed] [Google Scholar]
- Rowe M., Evans H. S., Young L. S., Hennessy K., Kieff E., Rickinson A. B. Monoclonal antibodies to the latent membrane protein of Epstein-Barr virus reveal heterogeneity of the protein and inducible expression in virus-transformed cells. J Gen Virol. 1987 Jun;68(Pt 6):1575–1586. doi: 10.1099/0022-1317-68-6-1575. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Saito I., Servenius B., Compton T., Fox R. I. Detection of Epstein-Barr virus DNA by polymerase chain reaction in blood and tissue biopsies from patients with Sjogren's syndrome. J Exp Med. 1989 Jun 1;169(6):2191–2198. doi: 10.1084/jem.169.6.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S. S., Sheibani K., Fishleder A., Ben-Ezra J., Bailey A., Koo C. H., Burke J. S., Tubbs R., Rappaport H. Monocytoid B-cell lymphoma in patients with Sjögren's syndrome: a clinicopathologic study of 13 patients. Hum Pathol. 1991 May;22(5):422–430. doi: 10.1016/0046-8177(91)90126-a. [DOI] [PubMed] [Google Scholar]
- Sixbey J. W., Shirley P., Chesney P. J., Buntin D. M., Resnick L. Detection of a second widespread strain of Epstein-Barr virus. Lancet. 1989 Sep 30;2(8666):761–765. doi: 10.1016/s0140-6736(89)90829-5. [DOI] [PubMed] [Google Scholar]
- Sixbey J. W., Shirley P., Sloas M., Raab-Traub N., Israele V. A transformation-incompetent, nuclear antigen 2-deleted Epstein-Barr virus associated with replicative infection. J Infect Dis. 1991 May;163(5):1008–1015. doi: 10.1093/infdis/163.5.1008. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Felix D. H., Wray D., Southam J. C., Cubie H. A., Crawford D. H. Epstein-Barr virus gene expression and epithelial cell differentiation in oral hairy leukoplakia. Am J Pathol. 1991 Dec;139(6):1369–1380. [PMC free article] [PubMed] [Google Scholar]
- Venables P. J., Teo C. G., Baboonian C., Griffin B. E., Hughes R. A. Persistence of Epstein-Barr virus in salivary gland biopsies from healthy individuals and patients with Sjögren's syndrome. Clin Exp Immunol. 1989 Mar;75(3):359–364. [PMC free article] [PubMed] [Google Scholar]
- Wang D., Liebowitz D., Wang F., Gregory C., Rickinson A., Larson R., Springer T., Kieff E. Epstein-Barr virus latent infection membrane protein alters the human B-lymphocyte phenotype: deletion of the amino terminus abolishes activity. J Virol. 1988 Nov;62(11):4173–4184. doi: 10.1128/jvi.62.11.4173-4184.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss L. M., Gaffey M. J., Shibata D. Lymphoepithelioma-like carcinoma and its relationship to Epstein-Barr virus. Am J Clin Pathol. 1991 Aug;96(2):156–158. doi: 10.1093/ajcp/96.2.156. [DOI] [PubMed] [Google Scholar]
- Weiss L. M., Movahed L. A. In situ demonstration of Epstein-Barr viral genomes in viral-associated B cell lymphoproliferations. Am J Pathol. 1989 Mar;134(3):651–659. [PMC free article] [PubMed] [Google Scholar]
- Whittingham S., McNeilage J., Mackay I. R. Primary Sjögren's syndrome after infectious mononucleosis. Ann Intern Med. 1985 Apr;102(4):490–493. doi: 10.7326/0003-4819-102-4-490. [DOI] [PubMed] [Google Scholar]
- Wieczorek R., Jakobiec F. A., Sacks E. H., Knowles D. M. The immunoarchitecture of the normal human lacrimal gland. Relevancy for understanding pathologic conditions. Ophthalmology. 1988 Jan;95(1):100–109. doi: 10.1016/s0161-6420(88)33228-8. [DOI] [PubMed] [Google Scholar]
- Wolf H., Haus M., Wilmes E. Persistence of Epstein-Barr virus in the parotid gland. J Virol. 1984 Sep;51(3):795–798. doi: 10.1128/jvi.51.3.795-798.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen M. T., Pflugfelder S. C., Crouse C. A., Atherton S. S. Cytoskeletal antigen expression in ocular mucosa-associated lymphoid tissue. Invest Ophthalmol Vis Sci. 1992 Oct;33(11):3235–3241. [PubMed] [Google Scholar]
- Young L. S., Lau R., Rowe M., Niedobitek G., Packham G., Shanahan F., Rowe D. T., Greenspan D., Greenspan J. S., Rickinson A. B. Differentiation-associated expression of the Epstein-Barr virus BZLF1 transactivator protein in oral hairy leukoplakia. J Virol. 1991 Jun;65(6):2868–2874. doi: 10.1128/jvi.65.6.2868-2874.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L., Alfieri C., Hennessy K., Evans H., O'Hara C., Anderson K. C., Ritz J., Shapiro R. S., Rickinson A., Kieff E. Expression of Epstein-Barr virus transformation-associated genes in tissues of patients with EBV lymphoproliferative disease. N Engl J Med. 1989 Oct 19;321(16):1080–1085. doi: 10.1056/NEJM198910193211604. [DOI] [PubMed] [Google Scholar]