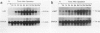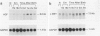Abstract
Hepatocyte growth factor (HGF) is a potent mitogen for mature hepatocytes in vitro. The receptor for HGF has recently been characterized as the product of the proto-oncogene c-met. We have examined the possible involvement of HGF in hepatic growth and differentiation in the rat. The experimental systems used were acetylaminofluorene treatment combined with partial hepatectomy to induce proliferation and differentiation of oval cells in adult liver and the pre- and postnatal liver. In the acetylaminofluorene model, Northern blot analysis showed that level of HGF transcripts increased one day after partial hepatectomy, reached a peak by day 6, were maintained at that level until day 13, and then declined, reaching normal level at 20 days. The expression of c-met also increased gradually, reached a peak around 9 to 13 days after partial hepatectomy, at which time oval cell proliferation was most prominent. In the developing liver, an elevated level of HGF transcripts was found between 4 and 21 days after birth. The expression of c-met also slightly increased at the same time. In situ hybridization showed that the transcripts for HGF were localized in desmin-positive Ito cells, whereas the transcripts for c-met were strongly expressed by oval cells. We have shown earlier that Ito cells and oval cells proliferate simultaneously and exist in close proximity in the acetylaminofluorene model and that Ito cells are a primary source of growth factors such as transforming growth factor-alpha and acidic fibroblast growth factors. The data presented here suggest that HGF is, in combination with other growth factors, involved in the proliferation and differentiation of oval cells via a paracrine mechanism.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bottaro D. P., Rubin J. S., Faletto D. L., Chan A. M., Kmiecik T. E., Vande Woude G. F., Aaronson S. A. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991 Feb 15;251(4995):802–804. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- DiPersio C. M., Jackson D. A., Zaret K. S. The extracellular matrix coordinately modulates liver transcription factors and hepatocyte morphology. Mol Cell Biol. 1991 Sep;11(9):4405–4414. doi: 10.1128/mcb.11.9.4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evarts R. P., Nagy P., Marsden E., Thorgeirsson S. S. A precursor-product relationship exists between oval cells and hepatocytes in rat liver. Carcinogenesis. 1987 Nov;8(11):1737–1740. doi: 10.1093/carcin/8.11.1737. [DOI] [PubMed] [Google Scholar]
- Evarts R. P., Nagy P., Marsden E., Thorgeirsson S. S. In situ hybridization studies on expression of albumin and alpha-fetoprotein during the early stage of neoplastic transformation in rat liver. Cancer Res. 1987 Oct 15;47(20):5469–5475. [PubMed] [Google Scholar]
- Evarts R. P., Nagy P., Nakatsukasa H., Marsden E., Thorgeirsson S. S. In vivo differentiation of rat liver oval cells into hepatocytes. Cancer Res. 1989 Mar 15;49(6):1541–1547. [PubMed] [Google Scholar]
- Evarts R. P., Nakatsukasa H., Marsden E. R., Hu Z., Thorgeirsson S. S. Expression of transforming growth factor-alpha in regenerating liver and during hepatic differentiation. Mol Carcinog. 1992;5(1):25–31. doi: 10.1002/mc.2940050107. [DOI] [PubMed] [Google Scholar]
- Fausto N., Mead J. E., Gruppuso P. A., Braun L. TGF-beta in liver development, regeneration, and carcinogenesis. Ann N Y Acad Sci. 1990;593:231–242. doi: 10.1111/j.1749-6632.1990.tb16115.x. [DOI] [PubMed] [Google Scholar]
- Giordano S., Ponzetto C., Di Renzo M. F., Cooper C. S., Comoglio P. M. Tyrosine kinase receptor indistinguishable from the c-met protein. Nature. 1989 May 11;339(6220):155–156. doi: 10.1038/339155a0. [DOI] [PubMed] [Google Scholar]
- Igawa T., Kanda S., Kanetake H., Saitoh Y., Ichihara A., Tomita Y., Nakamura T. Hepatocyte growth factor is a potent mitogen for cultured rabbit renal tubular epithelial cells. Biochem Biophys Res Commun. 1991 Jan 31;174(2):831–838. doi: 10.1016/0006-291x(91)91493-v. [DOI] [PubMed] [Google Scholar]
- Kan M., Huang J. S., Mansson P. E., Yasumitsu H., Carr B., McKeehan W. L. Heparin-binding growth factor type 1 (acidic fibroblast growth factor): a potential biphasic autocrine and paracrine regulator of hepatocyte regeneration. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7432–7436. doi: 10.1073/pnas.86.19.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan M., Zhang G. H., Zarnegar R., Michalopoulos G., Myoken Y., McKeehan W. L., Stevens J. I. Hepatocyte growth factor/hepatopoietin A stimulates the growth of rat kidney proximal tubule epithelial cells (RPTE), rat nonparenchymal liver cells, human melanoma cells, mouse keratinocytes and stimulates anchorage-independent growth of SV-40 transformed RPTE. Biochem Biophys Res Commun. 1991 Jan 15;174(1):331–337. doi: 10.1016/0006-291x(91)90524-b. [DOI] [PubMed] [Google Scholar]
- Kinoshita T., Hirao S., Matsumoto K., Nakamura T. Possible endocrine control by hepatocyte growth factor of liver regeneration after partial hepatectomy. Biochem Biophys Res Commun. 1991 May 31;177(1):330–335. doi: 10.1016/0006-291x(91)91987-n. [DOI] [PubMed] [Google Scholar]
- Konishi T., Takehara T., Tsuji T., Ohsato K., Matsumoto K., Nakamura T. Scatter factor from human embryonic lung fibroblasts is probably identical to hepatocyte growth factor. Biochem Biophys Res Commun. 1991 Oct 31;180(2):765–773. doi: 10.1016/s0006-291x(05)81131-3. [DOI] [PubMed] [Google Scholar]
- Liesveld J. L., Abboud C. N., Duerst R. E., Ryan D. H., Brennan J. K., Lichtman M. A. Characterization of human marrow stromal cells: role in progenitor cell binding and granulopoiesis. Blood. 1989 May 15;73(7):1794–1800. [PubMed] [Google Scholar]
- Lindroos P. M., Zarnegar R., Michalopoulos G. K. Hepatocyte growth factor (hepatopoietin A) rapidly increases in plasma before DNA synthesis and liver regeneration stimulated by partial hepatectomy and carbon tetrachloride administration. Hepatology. 1991 Apr;13(4):743–750. [PubMed] [Google Scholar]
- Loreal O., Levavasseur F., Rescan P. Y., Yamada Y., Guillouzo A., Clement B. Differential expression of laminin chains in hepatic lipocytes. FEBS Lett. 1991 Sep 23;290(1-2):9–12. doi: 10.1016/0014-5793(91)81213-r. [DOI] [PubMed] [Google Scholar]
- Marsden E. R., Hu Z., Fujio K., Nakatsukasa H., Thorgeirsson S. S., Evarts R. P. Expression of acidic fibroblast growth factor in regenerating liver and during hepatic differentiation. Lab Invest. 1992 Oct;67(4):427–433. [PubMed] [Google Scholar]
- Matsumoto K., Hashimoto K., Yoshikawa K., Nakamura T. Marked stimulation of growth and motility of human keratinocytes by hepatocyte growth factor. Exp Cell Res. 1991 Sep;196(1):114–120. doi: 10.1016/0014-4827(91)90462-4. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Nakamura T. Hepatocyte growth factor: molecular structure, roles in liver regeneration, and other biological functions. Crit Rev Oncog. 1992;3(1-2):27–54. [PubMed] [Google Scholar]
- Matsumoto K., Tajima H., Nakamura T. Hepatocyte growth factor is a potent stimulator of human melanocyte DNA synthesis and growth. Biochem Biophys Res Commun. 1991 Apr 15;176(1):45–51. doi: 10.1016/0006-291x(91)90887-d. [DOI] [PubMed] [Google Scholar]
- Mead J. E., Fausto N. Transforming growth factor alpha may be a physiological regulator of liver regeneration by means of an autocrine mechanism. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1558–1562. doi: 10.1073/pnas.86.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulos G. K., Zarnegav R. Hepatocyte growth factor. Hepatology. 1992 Jan;15(1):149–155. doi: 10.1002/hep.1840150125. [DOI] [PubMed] [Google Scholar]
- Michalopoulos G., Houck K. A., Dolan M. L., Leutteke N. C. Control of hepatocyte replication by two serum factors. Cancer Res. 1984 Oct;44(10):4414–4419. [PubMed] [Google Scholar]
- Montesano R., Matsumoto K., Nakamura T., Orci L. Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell. 1991 Nov 29;67(5):901–908. doi: 10.1016/0092-8674(91)90363-4. [DOI] [PubMed] [Google Scholar]
- Nagy P., Evarts R. P., McMahon J. B., Thorgeirsson S. S. Role of TGF-beta in normal differentiation and oncogenesis in rat liver. Mol Carcinog. 1989;2(6):345–354. doi: 10.1002/mc.2940020609. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Nawa K., Ichihara A. Partial purification and characterization of hepatocyte growth factor from serum of hepatectomized rats. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1450–1459. doi: 10.1016/0006-291x(84)91253-1. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Nishizawa T., Hagiya M., Seki T., Shimonishi M., Sugimura A., Tashiro K., Shimizu S. Molecular cloning and expression of human hepatocyte growth factor. Nature. 1989 Nov 23;342(6248):440–443. doi: 10.1038/342440a0. [DOI] [PubMed] [Google Scholar]
- Nakatsukasa H., Nagy P., Evarts R. P., Hsia C. C., Marsden E., Thorgeirsson S. S. Cellular distribution of transforming growth factor-beta 1 and procollagen types I, III, and IV transcripts in carbon tetrachloride-induced rat liver fibrosis. J Clin Invest. 1990 Jun;85(6):1833–1843. doi: 10.1172/JCI114643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldini L., Vigna E., Narsimhan R. P., Gaudino G., Zarnegar R., Michalopoulos G. K., Comoglio P. M. Hepatocyte growth factor (HGF) stimulates the tyrosine kinase activity of the receptor encoded by the proto-oncogene c-MET. Oncogene. 1991 Apr;6(4):501–504. [PubMed] [Google Scholar]
- Naldini L., Weidner K. M., Vigna E., Gaudino G., Bardelli A., Ponzetto C., Narsimhan R. P., Hartmann G., Zarnegar R., Michalopoulos G. K. Scatter factor and hepatocyte growth factor are indistinguishable ligands for the MET receptor. EMBO J. 1991 Oct;10(10):2867–2878. doi: 10.1002/j.1460-2075.1991.tb07836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noji S., Tashiro K., Koyama E., Nohno T., Ohyama K., Taniguchi S., Nakamura T. Expression of hepatocyte growth factor gene in endothelial and Kupffer cells of damaged rat livers, as revealed by in situ hybridization. Biochem Biophys Res Commun. 1990 Nov 30;173(1):42–47. doi: 10.1016/s0006-291x(05)81018-6. [DOI] [PubMed] [Google Scholar]
- Ogawa K., Medline A., Farber E. Sequential analysis of hepatic carcinogenesis: the comparative architecture of preneoplastic, malignant, prenatal, postnatal and regenerating liver. Br J Cancer. 1979 Nov;40(5):782–790. doi: 10.1038/bjc.1979.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadori G., Neubauer K., Odenthal M., Nakamura T., Knittel T., Schwögler S., Meyer zum Büschenfelde K. H. The gene of hepatocyte growth factor is expressed in fat-storing cells of rat liver and is downregulated during cell growth and by transforming growth factor-beta. Biochem Biophys Res Commun. 1992 Mar 16;183(2):739–742. doi: 10.1016/0006-291x(92)90545-v. [DOI] [PubMed] [Google Scholar]
- Reid L. M. Stem cell biology, hormone/matrix synergies and liver differentiation. Curr Opin Cell Biol. 1990 Feb;2(1):121–130. doi: 10.1016/s0955-0674(05)80042-0. [DOI] [PubMed] [Google Scholar]
- Sell S. Is there a liver stem cell? Cancer Res. 1990 Jul 1;50(13):3811–3815. [PubMed] [Google Scholar]
- Stoker M., Perryman M. An epithelial scatter factor released by embryo fibroblasts. J Cell Sci. 1985 Aug;77:209–223. doi: 10.1242/jcs.77.1.209. [DOI] [PubMed] [Google Scholar]
- Tashiro K., Hagiya M., Nishizawa T., Seki T., Shimonishi M., Shimizu S., Nakamura T. Deduced primary structure of rat hepatocyte growth factor and expression of the mRNA in rat tissues. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3200–3204. doi: 10.1073/pnas.87.8.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner K. M., Arakaki N., Hartmann G., Vandekerckhove J., Weingart S., Rieder H., Fonatsch C., Tsubouchi H., Hishida T., Daikuhara Y. Evidence for the identity of human scatter factor and human hepatocyte growth factor. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7001–7005. doi: 10.1073/pnas.88.16.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf H. K., Zarnegar R., Michalopoulos G. K. Localization of hepatocyte growth factor in human and rat tissues: an immunohistochemical study. Hepatology. 1991 Sep;14(3):488–494. [PubMed] [Google Scholar]
- Zarnegar R., DeFrances M. C., Kost D. P., Lindroos P., Michalopoulos G. K. Expression of hepatocyte growth factor mRNA in regenerating rat liver after partial hepatectomy. Biochem Biophys Res Commun. 1991 May 31;177(1):559–565. doi: 10.1016/0006-291x(91)92020-k. [DOI] [PubMed] [Google Scholar]