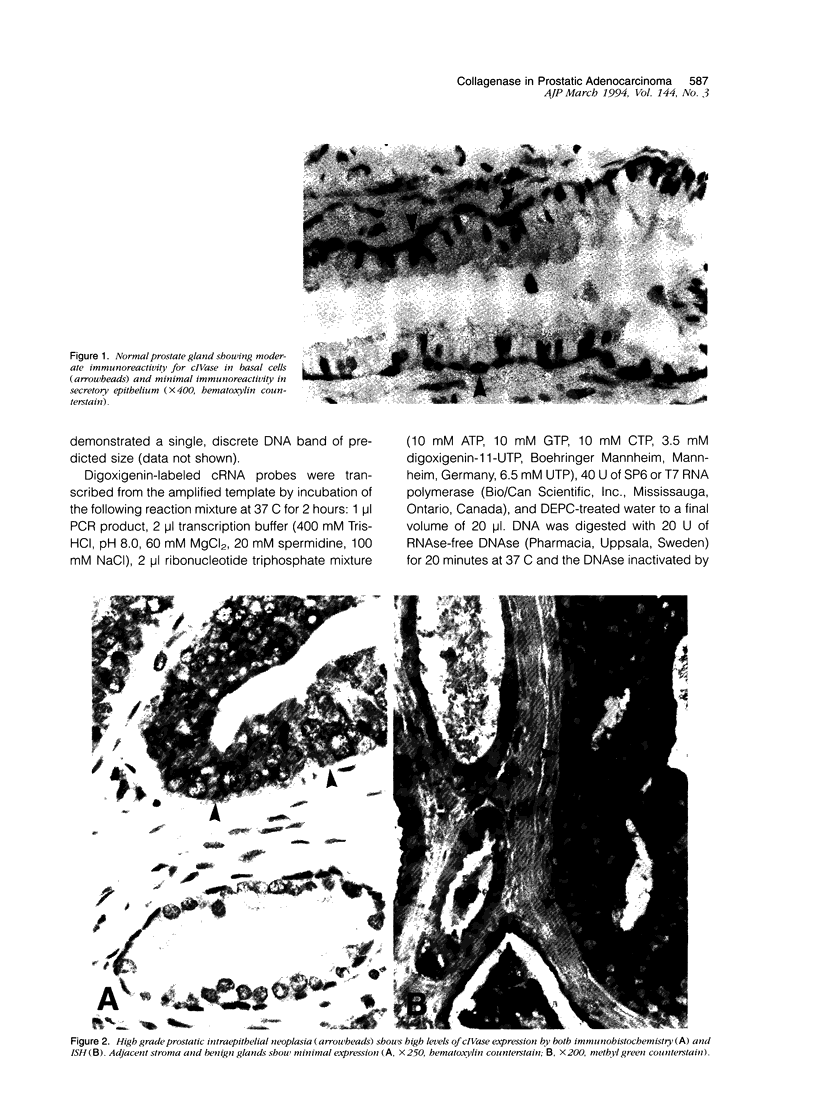
Abstract
The expression of the 72-kd type IV collagenase has been implicated as an important factor in determining the invasive potential of malignant tumors. Using immunohistochemistry and nonisotopic in situ hybridization, type IV collagenase expression was assessed in benign and malignant prostatic tissue obtained from 117 surgical and autopsy specimens. Diffuse strong staining for type IV collagenase mRNA and protein was identified in the malignant cells of more than 85% of prostatic adenocarcinomas and the dysplastic cells of high grade prostatic intraepithelial neoplasia. Benign hyperplastic epithelium showed moderate expression in basal cells and mild expression in secretory cells. The qualitative patterns of type IV collagenase expression in prostatic epithelium at the protein and mRNA levels in individual cases were identical. There was no correlation between the level of type IV collagenase expression and either tumor grade or stage. In 10% of adenocarcinomas, focal mild to moderate stromal cell immunoreactivity was present but mRNA was not detectable in the stromal compartment in any case. The enhanced expression of type IV collagenase in dysplastic epithelium and prostatic adenocarcinoma suggests it contributes to the development of the invasive phenotype. The vast majority of the enzyme present in these tumors is synthesized by malignant cells and its production by stromal cells is negligible.
Full text
PDF

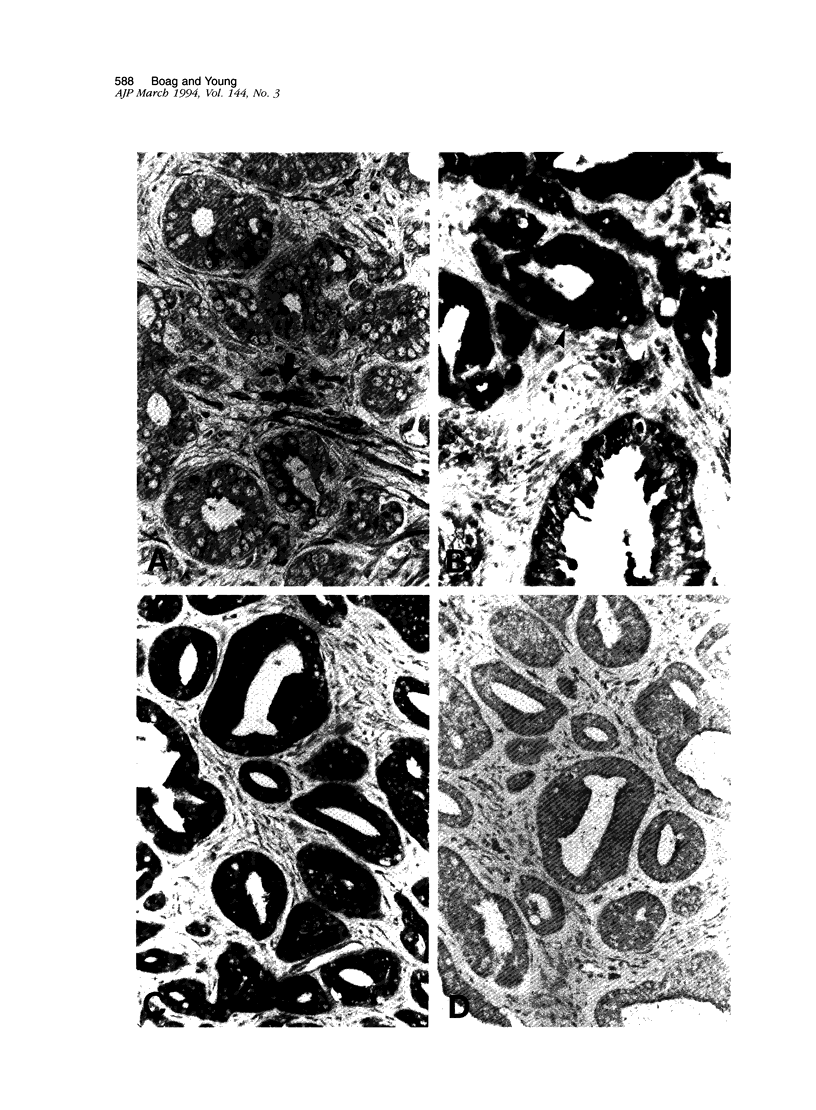



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boag A. H., Young I. D. Immunohistochemical analysis of type IV collagenase expression in prostatic hyperplasia and adenocarcinoma. Mod Pathol. 1993 Jan;6(1):65–68. [PubMed] [Google Scholar]
- Campo E., Merino M. J., Tavassoli F. A., Charonis A. S., Stetler-Stevenson W. G., Liotta L. A. Evaluation of basement membrane components and the 72 kDa type IV collagenase in serous tumors of the ovary. Am J Surg Pathol. 1992 May;16(5):500–507. doi: 10.1097/00000478-199205000-00009. [DOI] [PubMed] [Google Scholar]
- Collier I. E., Bruns G. A., Goldberg G. I., Gerhard D. S. On the structure and chromosome location of the 72- and 92-kDa human type IV collagenase genes. Genomics. 1991 Mar;9(3):429–434. doi: 10.1016/0888-7543(91)90408-7. [DOI] [PubMed] [Google Scholar]
- Collier I. E., Wilhelm S. M., Eisen A. Z., Marmer B. L., Grant G. A., Seltzer J. L., Kronberger A., He C. S., Bauer E. A., Goldberg G. I. H-ras oncogene-transformed human bronchial epithelial cells (TBE-1) secrete a single metalloprotease capable of degrading basement membrane collagen. J Biol Chem. 1988 May 15;263(14):6579–6587. [PubMed] [Google Scholar]
- Fuchs M. E., Brawer M. K., Rennels M. A., Nagle R. B. The relationship of basement membrane to histologic grade of human prostatic carcinoma. Mod Pathol. 1989 Mar;2(2):105–111. [PubMed] [Google Scholar]
- Grigioni W. F., Garbisa S., D'Errico A., Baccarini P., Stetler-Stevenson W. G., Liotta L. A., Mancini A. M. Evaluation of hepatocellular carcinoma aggressiveness by a panel of extracellular matrix antigens. Am J Pathol. 1991 Mar;138(3):647–654. [PMC free article] [PubMed] [Google Scholar]
- Howard E. W., Bullen E. C., Banda M. J. Preferential inhibition of 72- and 92-kDa gelatinases by tissue inhibitor of metalloproteinases-2. J Biol Chem. 1991 Jul 15;266(20):13070–13075. [PubMed] [Google Scholar]
- Levy A. T., Cioce V., Sobel M. E., Garbisa S., Grigioni W. F., Liotta L. A., Stetler-Stevenson W. G. Increased expression of the Mr 72,000 type IV collagenase in human colonic adenocarcinoma. Cancer Res. 1991 Jan 1;51(1):439–444. [PubMed] [Google Scholar]
- Liotta L. A., Steeg P. S., Stetler-Stevenson W. G. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991 Jan 25;64(2):327–336. doi: 10.1016/0092-8674(91)90642-c. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M. The matrix-degrading metalloproteinases. Bioessays. 1992 Jul;14(7):455–463. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- McNeal J. E., Bostwick D. G. Intraductal dysplasia: a premalignant lesion of the prostate. Hum Pathol. 1986 Jan;17(1):64–71. doi: 10.1016/s0046-8177(86)80156-3. [DOI] [PubMed] [Google Scholar]
- Monteagudo C., Merino M. J., San-Juan J., Liotta L. A., Stetler-Stevenson W. G. Immunohistochemical distribution of type IV collagenase in normal, benign, and malignant breast tissue. Am J Pathol. 1990 Mar;136(3):585–592. [PMC free article] [PubMed] [Google Scholar]
- Pajouh M. S., Nagle R. B., Breathnach R., Finch J. S., Brawer M. K., Bowden G. T. Expression of metalloproteinase genes in human prostate cancer. J Cancer Res Clin Oncol. 1991;117(2):144–150. doi: 10.1007/BF01613138. [DOI] [PubMed] [Google Scholar]
- Poulsom R., Pignatelli M., Stetler-Stevenson W. G., Liotta L. A., Wright P. A., Jeffery R. E., Longcroft J. M., Rogers L., Stamp G. W. Stromal expression of 72 kda type IV collagenase (MMP-2) and TIMP-2 mRNAs in colorectal neoplasia. Am J Pathol. 1992 Aug;141(2):389–396. [PMC free article] [PubMed] [Google Scholar]
- Pyke C., Ralfkiaer E., Huhtala P., Hurskainen T., Danø K., Tryggvason K. Localization of messenger RNA for Mr 72,000 and 92,000 type IV collagenases in human skin cancers by in situ hybridization. Cancer Res. 1992 Mar 1;52(5):1336–1341. [PubMed] [Google Scholar]
- Soini Y., Päkkö P., Autio-Harmainen H. Genes of laminin B1 chain, alpha 1 (IV) chain of type IV collagen, and 72-kd type IV collagenase are mainly expressed by the stromal cells of lung carcinomas. Am J Pathol. 1993 May;142(5):1622–1630. [PMC free article] [PubMed] [Google Scholar]
- Stearns M. E., Wang M. Type IV collagenase (M(r) 72,000) expression in human prostate: benign and malignant tissue. Cancer Res. 1993 Feb 15;53(4):878–883. [PubMed] [Google Scholar]
- Stetler-Stevenson W. G. Type IV collagenases in tumor invasion and metastasis. Cancer Metastasis Rev. 1990 Dec;9(4):289–303. doi: 10.1007/BF00049520. [DOI] [PubMed] [Google Scholar]
- Woessner J. F., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991 May;5(8):2145–2154. [PubMed] [Google Scholar]
- Young I. D., Ailles L., Deugau K., Kisilevsky R. Transcription of cRNA for in situ hybridization from polymerase chain reaction-amplified DNA. Lab Invest. 1991 May;64(5):709–712. [PubMed] [Google Scholar]