Abstract
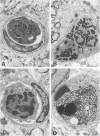
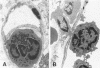
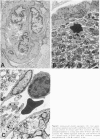
The results of several experimental studies of focal ischemia and anecdotal observations suggest that leukocytes may contribute to the injury initiated by an arterial occlusion. The timing and the nature of leukocyte responses in evolving brain infarcts (either human or experimental) are incompletely characterized. This is a study of experimental brain lesions in 96 Wistar rats that underwent occlusion of a large intracranial artery for variable intervals ranging between 30 minutes and 7 days. The experimental model, based on the occlusion of a middle cerebral artery ostium via the insertion of a nylon monofilament through the external carotid artery, does not require opening the skull; therefore, the inflammatory response is not influenced by the effects of craniotomy and changes in intracranial pressure are only those induced by the ischemic lesion. All 96 animals having the same type of arterial occlusion developed an ischemic brain lesion (limited to the territory of the corresponding artery) that evolved into an area of extensive neuronal necrosis over a period of 6 to 12 hours followed by pan-necrosis (infarct) approximately 60 hours later. In this study, leukocytes (in particular polymorphonuclear cells) were detected in the microvessels (capillaries and venules) of the ischemic hemisphere as early as 30 minutes after the arterial occlusion. Numbers of intravascular neutrophils peaked at 12 hours, whereas intraparenchymal granulocytes were most numerous at 24 hours; a few granulocytes were visible in the brain infarct as late as day 7. Circulating monocytes were first detected within the capillaries/venules of the ischemic area after 4 to 6 hours. Platelet aggregates were more abundant in the arterial than the venous side of the circulation, and luminal obstruction of arteries by platelet aggregates became noticeable only 48 hours after the arterial occlusion. Fibrin thrombi were conspicuous for their absence. These observations provide the background for studies that will attempt to unravel the relationship between the biological responses of leukocytes and neuronal necrosis secondary to focal ischemia.
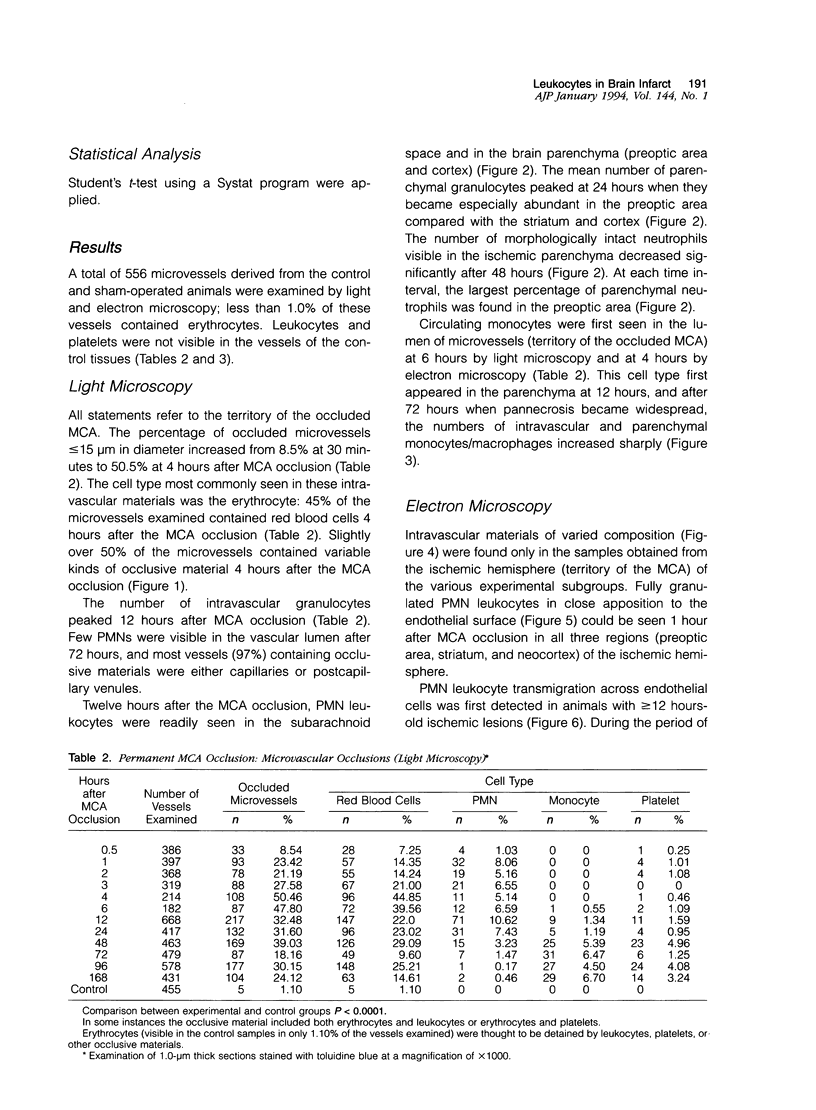
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bamford J., Dennis M., Sandercock P., Burn J., Warlow C. The frequency, causes and timing of death within 30 days of a first stroke: the Oxfordshire Community Stroke Project. J Neurol Neurosurg Psychiatry. 1990 Oct;53(10):824–829. doi: 10.1136/jnnp.53.10.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone F. C., Hillegass L. M., Price W. J., White R. F., Lee E. V., Feuerstein G. Z., Sarau H. M., Clark R. K., Griswold D. E. Polymorphonuclear leukocyte infiltration into cerebral focal ischemic tissue: myeloperoxidase activity assay and histologic verification. J Neurosci Res. 1991 Jul;29(3):336–345. doi: 10.1002/jnr.490290309. [DOI] [PubMed] [Google Scholar]
- Chuaqui R., Tapia J. Histologic assessment of the age of recent brain infarcts in man. J Neuropathol Exp Neurol. 1993 Sep;52(5):481–489. doi: 10.1097/00005072-199309000-00006. [DOI] [PubMed] [Google Scholar]
- Dorovini-Zis K., Bowman P. D., Prameya R. Adhesion and migration of human polymorphonuclear leukocytes across cultured bovine brain microvessel endothelial cells. J Neuropathol Exp Neurol. 1992 Mar;51(2):194–205. doi: 10.1097/00005072-199203000-00009. [DOI] [PubMed] [Google Scholar]
- Ernst E., Matrai A., Paulsen F. Leukocyte rheology in recent stroke. Stroke. 1987 Jan-Feb;18(1):59–62. doi: 10.1161/01.str.18.1.59. [DOI] [PubMed] [Google Scholar]
- Garcia J. H., Yoshida Y., Chen H., Li Y., Zhang Z. G., Lian J., Chen S., Chopp M. Progression from ischemic injury to infarct following middle cerebral artery occlusion in the rat. Am J Pathol. 1993 Feb;142(2):623–635. [PMC free article] [PubMed] [Google Scholar]
- Hernandez L. A., Grisham M. B., Twohig B., Arfors K. E., Harlan J. M., Granger D. N. Role of neutrophils in ischemia-reperfusion-induced microvascular injury. Am J Physiol. 1987 Sep;253(3 Pt 2):H699–H703. doi: 10.1152/ajpheart.1987.253.3.H699. [DOI] [PubMed] [Google Scholar]
- Jennings R. B., Reimer K. A. The cell biology of acute myocardial ischemia. Annu Rev Med. 1991;42:225–246. doi: 10.1146/annurev.me.42.020191.001301. [DOI] [PubMed] [Google Scholar]
- Klygis L. M., Reisberg B. E. Spinal epidural abscess caused by group G streptococci. Am J Med. 1991 Jul;91(1):89–90. doi: 10.1016/0002-9343(91)90078-c. [DOI] [PubMed] [Google Scholar]
- Laing R. J., Jakubowski J., Laing R. W. Middle cerebral artery occlusion without craniectomy in rats. Which method works best? Stroke. 1993 Feb;24(2):294–298. doi: 10.1161/01.str.24.2.294. [DOI] [PubMed] [Google Scholar]
- Longa E. Z., Weinstein P. R., Carlson S., Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989 Jan;20(1):84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- McEver R. P. Selectins: novel receptors that mediate leukocyte adhesion during inflammation. Thromb Haemost. 1991 Mar 4;65(3):223–228. [PubMed] [Google Scholar]
- Minematsu K., Li L., Fisher M., Sotak C. H., Davis M. A., Fiandaca M. S. Diffusion-weighted magnetic resonance imaging: rapid and quantitative detection of focal brain ischemia. Neurology. 1992 Jan;42(1):235–240. doi: 10.1212/wnl.42.1.235. [DOI] [PubMed] [Google Scholar]
- Mori E., del Zoppo G. J., Chambers J. D., Copeland B. R., Arfors K. E. Inhibition of polymorphonuclear leukocyte adherence suppresses no-reflow after focal cerebral ischemia in baboons. Stroke. 1992 May;23(5):712–718. doi: 10.1161/01.str.23.5.712. [DOI] [PubMed] [Google Scholar]
- Mulligan M. S., Polley M. J., Bayer R. J., Nunn M. F., Paulson J. C., Ward P. A. Neutrophil-dependent acute lung injury. Requirement for P-selectin (GMP-140). J Clin Invest. 1992 Oct;90(4):1600–1607. doi: 10.1172/JCI116029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa H., Kogure K. Correlation between cerebral blood flow and histologic changes in a new rat model of middle cerebral artery occlusion. Stroke. 1989 Aug;20(8):1037–1043. doi: 10.1161/01.str.20.8.1037. [DOI] [PubMed] [Google Scholar]
- Patarroyo M. Leukocyte adhesion in host defense and tissue injury. Clin Immunol Immunopathol. 1991 Sep;60(3):333–348. doi: 10.1016/0090-1229(91)90091-n. [DOI] [PubMed] [Google Scholar]
- Pozzilli C., Lenzi G. L., Argentino C., Carolei A., Rasura M., Signore A., Bozzao L., Pozzilli P. Imaging of leukocytic infiltration in human cerebral infarcts. Stroke. 1985 Mar-Apr;16(2):251–255. doi: 10.1161/01.str.16.2.251. [DOI] [PubMed] [Google Scholar]
- Prentice R. L., Szatrowski T. P., Kato H., Mason M. W. Leukocyte counts and cerebrovascular disease. J Chronic Dis. 1982;35(9):703–714. doi: 10.1016/0021-9681(82)90094-7. [DOI] [PubMed] [Google Scholar]
- Risau W., Wolburg H. Development of the blood-brain barrier. Trends Neurosci. 1990 May;13(5):174–178. doi: 10.1016/0166-2236(90)90043-a. [DOI] [PubMed] [Google Scholar]
- Schmid-Schönbein G. W. Capillary plugging by granulocytes and the no-reflow phenomenon in the microcirculation. Fed Proc. 1987 May 15;46(7):2397–2401. [PubMed] [Google Scholar]
- Schmid-Schönbein G. W., Engler R. L. Granulocytes as active participants in acute myocardial ischemia and infarction. Am J Cardiovasc Pathol. 1987 Jan;1(1):15–30. [PubMed] [Google Scholar]
- Silver F. L., Norris J. W., Lewis A. J., Hachinski V. C. Early mortality following stroke: a prospective review. Stroke. 1984 May-Jun;15(3):492–496. doi: 10.1161/01.str.15.3.492. [DOI] [PubMed] [Google Scholar]
- Sörnäs R., Ostlund H., Müller R. Cerebrospinal fluid cytology after stroke. Arch Neurol. 1972 Jun;26(6):489–501. doi: 10.1001/archneur.1972.00490120029002. [DOI] [PubMed] [Google Scholar]
- Wang P. Y., Kao C. H., Mui M. Y., Wang S. J. Leukocyte infiltration in acute hemispheric ischemic stroke. Stroke. 1993 Feb;24(2):236–240. doi: 10.1161/01.str.24.2.236. [DOI] [PubMed] [Google Scholar]
- del Zoppo G. J., Schmid-Schönbein G. W., Mori E., Copeland B. R., Chang C. M. Polymorphonuclear leukocytes occlude capillaries following middle cerebral artery occlusion and reperfusion in baboons. Stroke. 1991 Oct;22(10):1276–1283. doi: 10.1161/01.str.22.10.1276. [DOI] [PubMed] [Google Scholar]