Abstract
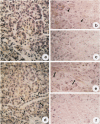
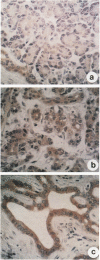
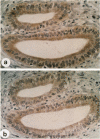
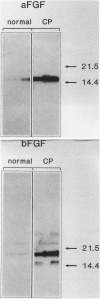
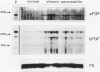
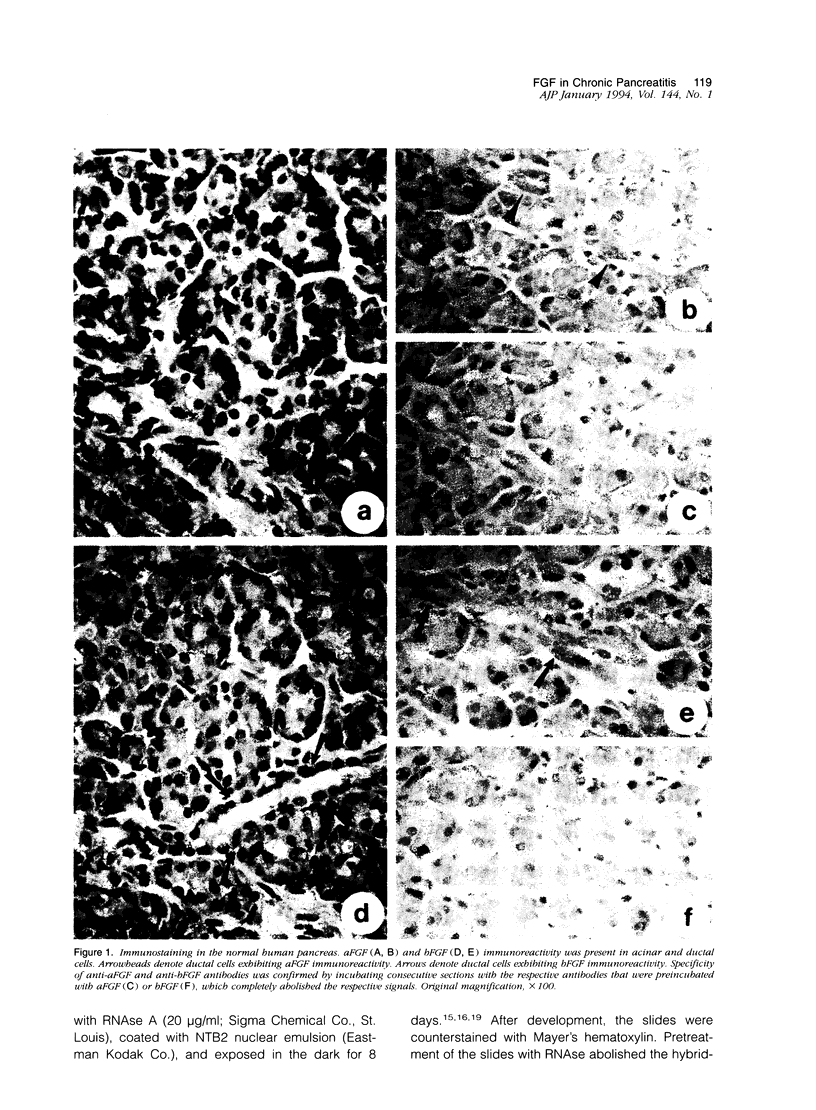
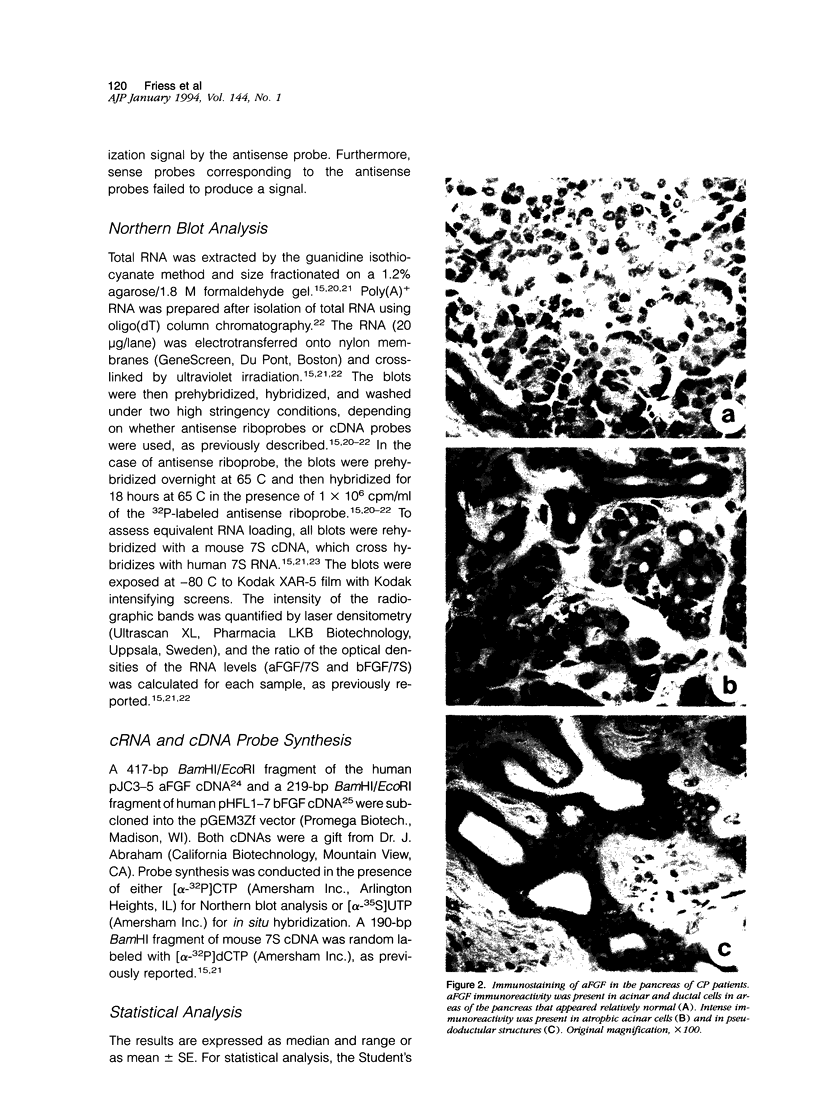
Acidic fibroblast growth factor (aFGF) and basic fibroblast growth factor (bFGF) belong to a family of mitogenic polypeptides that are involved in cellular proliferation and differentiation. In this study we investigated the potential role of aFGF and bFGF in chronic pancreatitis (CP), a fibrotic condition associated with acinar cell dedifferentiation and atrophy, and fibroblastic proliferation. By immunohistochemistry, aFGF and bFGF were abundant in pancreatic ductal and acinar cells in pancreatic tissues from CP patients. Immunoblotting with the same highly specific monoclonal antibodies demonstrated a marked increase in aFGF and bFGF in pancreatic homogenates from CP patients by comparison with the normal pancreas. Northern blot analysis indicated that, by comparison with normal controls, 16 of 21 CP tissues exhibited a 14-fold increase in aFGF mRNA levels, and 19 of 21 CP tissues exhibited a 15-fold increase in bFGF mRNA levels. In situ hybridization confirmed that this overexpression occurred in ductal and acinar cells, and indicated that both mRNA moieties colocalized with their respective proteins. These findings suggest that aFGF and bFGF may either be involved in the pathobiological mechanisms that occur in CP, or that their overexpression may be the consequence of other perturbations that occur in this disorder.
Full text
PDF



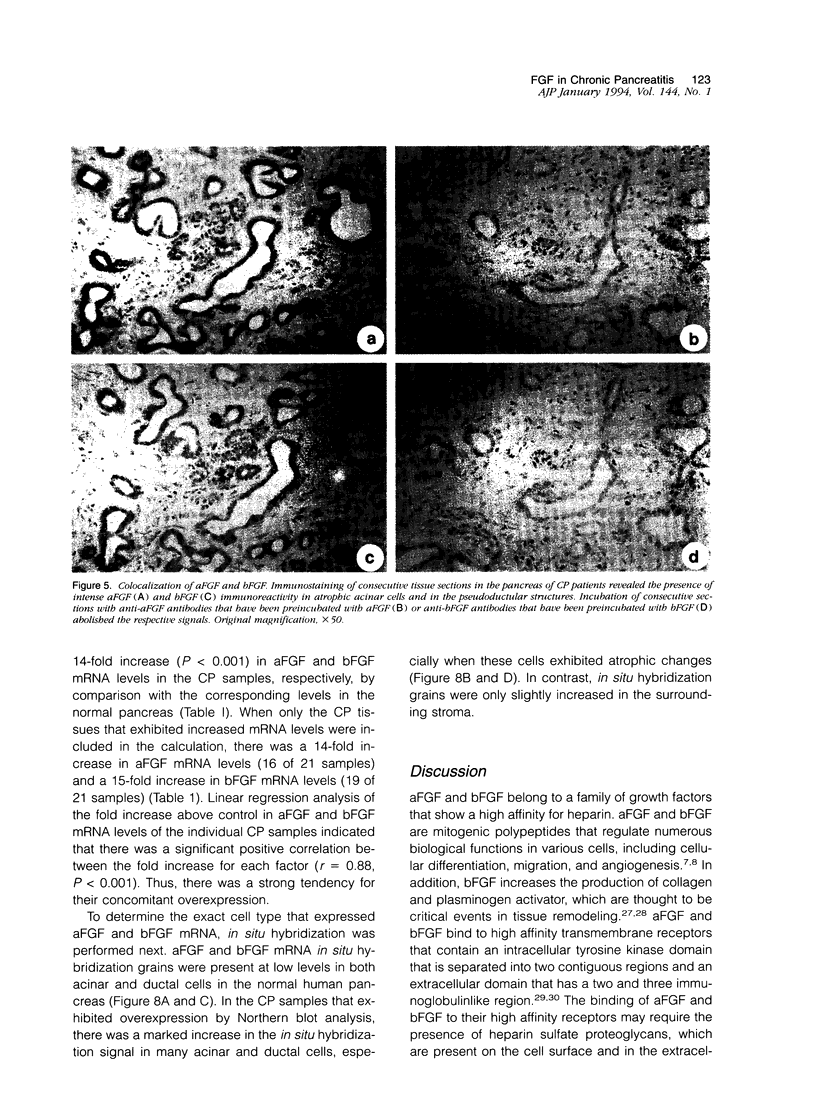
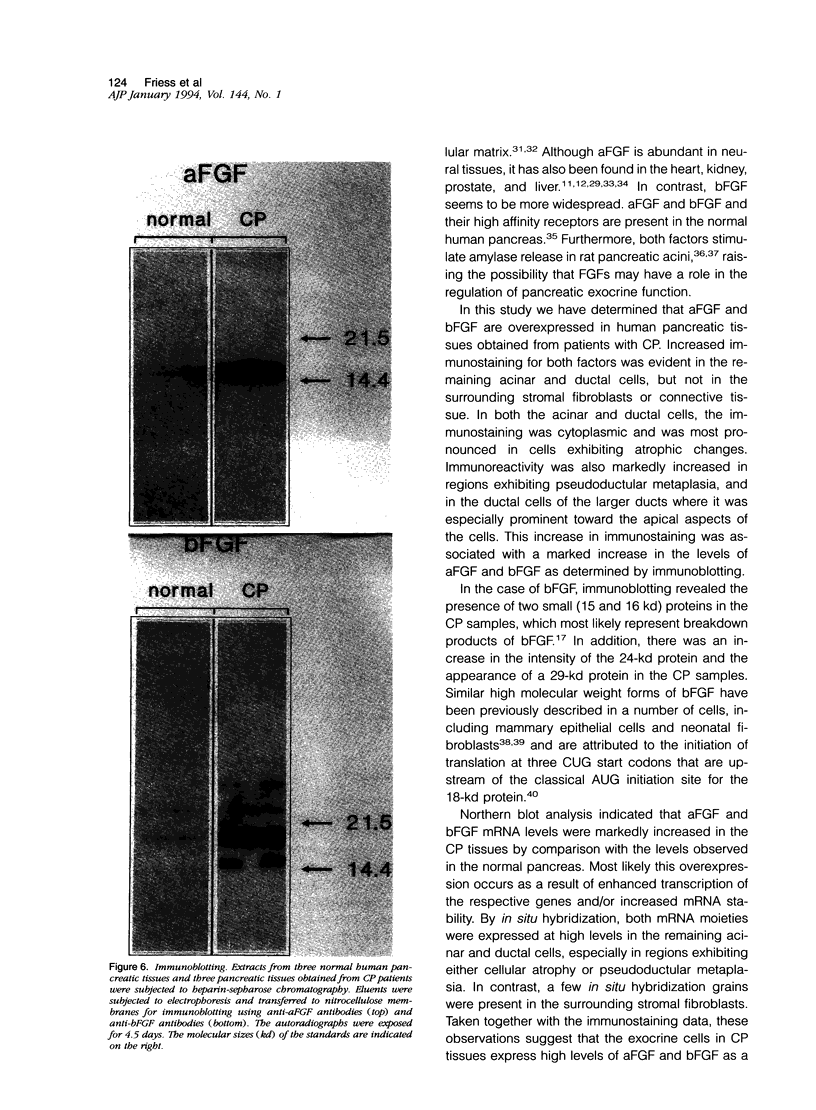
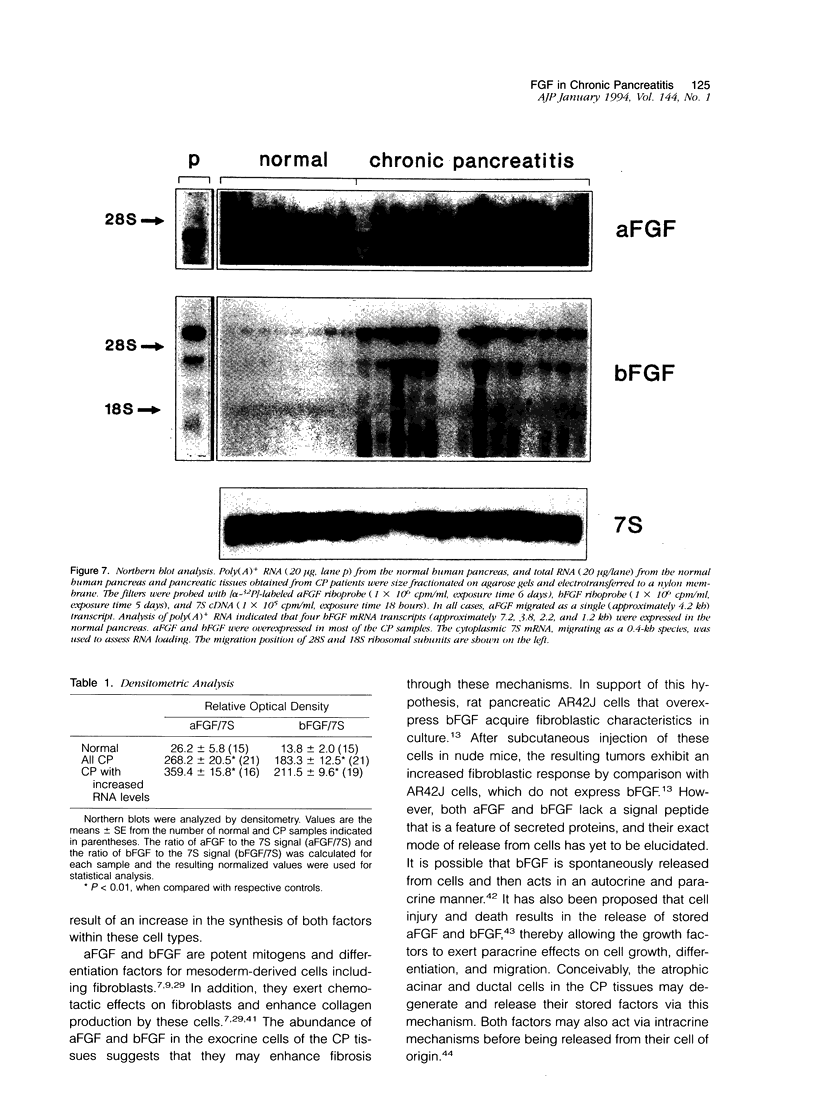
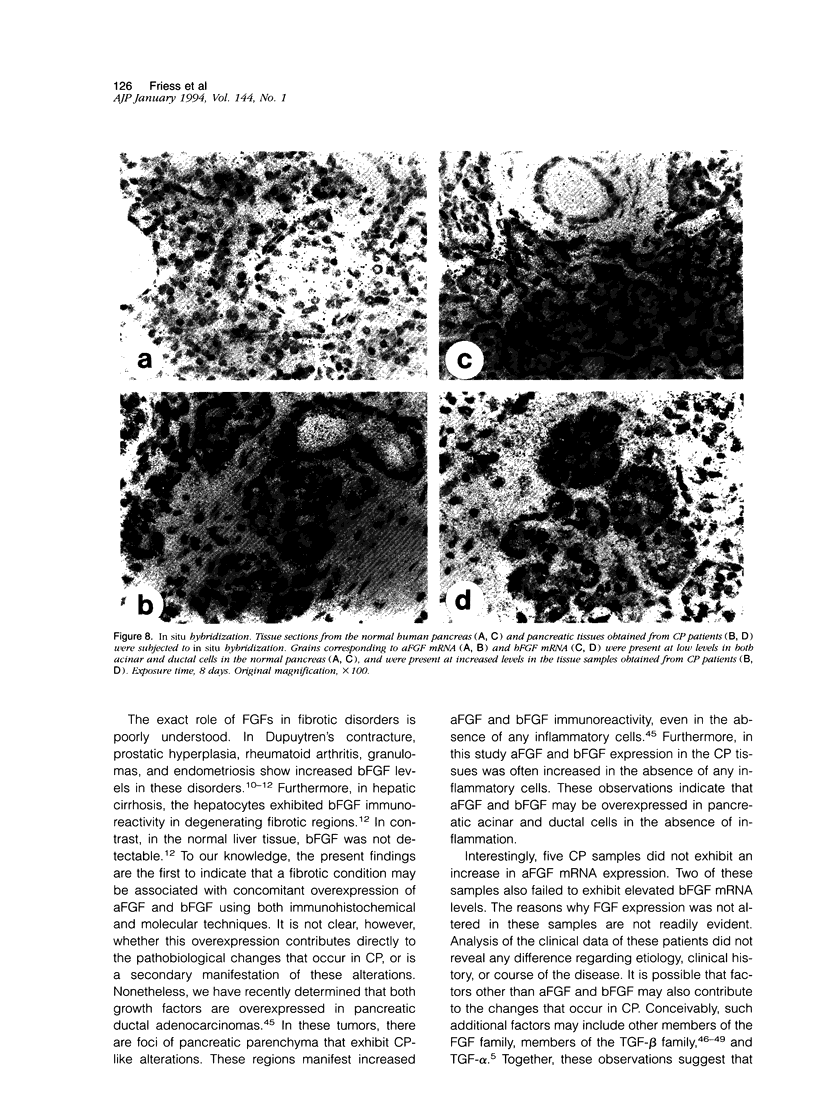


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham J. A., Whang J. L., Tumolo A., Mergia A., Friedman J., Gospodarowicz D., Fiddes J. C. Human basic fibroblast growth factor: nucleotide sequence and genomic organization. EMBO J. 1986 Oct;5(10):2523–2528. doi: 10.1002/j.1460-2075.1986.tb04530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin R. L., Korc M. Growth inhibition of human pancreatic carcinoma cells by transforming growth factor beta-1. Growth Factors. 1993;8(1):23–34. doi: 10.3109/08977199309029131. [DOI] [PubMed] [Google Scholar]
- Balmain A., Krumlauf R., Vass J. K., Birnie G. D. Cloning and characterisation of the abundant cytoplasmic 7S RNA from mouse cells. Nucleic Acids Res. 1982 Jul 24;10(14):4259–4277. doi: 10.1093/nar/10.14.4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beger H. G., Büchler M. Duodenum-preserving resection of the head of the pancreas in chronic pancreatitis with inflammatory mass in the head. World J Surg. 1990 Jan-Feb;14(1):83–87. doi: 10.1007/BF01670550. [DOI] [PubMed] [Google Scholar]
- Bockman D. E., Merlino G. Cytological changes in the pancreas of transgenic mice overexpressing transforming growth factor alpha. Gastroenterology. 1992 Dec;103(6):1883–1892. doi: 10.1016/0016-5085(92)91448-d. [DOI] [PubMed] [Google Scholar]
- Burgess W. H., Maciag T. The heparin-binding (fibroblast) growth factor family of proteins. Annu Rev Biochem. 1989;58:575–606. doi: 10.1146/annurev.bi.58.070189.003043. [DOI] [PubMed] [Google Scholar]
- Chandrasekar B., Korc M. Basic fibroblast growth factor is a calcium-mobilizing secretagogue in rat pancreatic acini. Biochem Biophys Res Commun. 1991 May 31;177(1):166–170. doi: 10.1016/0006-291x(91)91963-d. [DOI] [PubMed] [Google Scholar]
- Courty J., Loret C., Chevallier B., Moenner M., Barritault D. Biochemical comparative studies between eye- and brain-derived growth factors. Biochimie. 1987 May;69(5):511–516. doi: 10.1016/0300-9084(87)90088-5. [DOI] [PubMed] [Google Scholar]
- Dionne C. A., Crumley G., Bellot F., Kaplow J. M., Searfoss G., Ruta M., Burgess W. H., Jaye M., Schlessinger J. Cloning and expression of two distinct high-affinity receptors cross-reacting with acidic and basic fibroblast growth factors. EMBO J. 1990 Sep;9(9):2685–2692. doi: 10.1002/j.1460-2075.1990.tb07454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsässer H. P., Adler G., Kern H. F. Fibroblast structure and function during regeneration from hormone-induced acute pancreatitis in the rat. Pancreas. 1989;4(2):169–178. doi: 10.1097/00006676-198904000-00005. [DOI] [PubMed] [Google Scholar]
- Estival A., Louvel D., Couderc B., Prats H., Hollande E., Vaysse N., Clémente F. Morphological and biological modifications induced in a rat pancreatic acinar cancer cell line (AR4-2J) by unscheduled expression of basic fibroblast growth factors. Cancer Res. 1993 Mar 1;53(5):1182–1187. [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Fu Y. M., Spirito P., Yu Z. X., Biro S., Sasse J., Lei J., Ferrans V. J., Epstein S. E., Casscells W. Acidic fibroblast growth factor in the developing rat embryo. J Cell Biol. 1991 Sep;114(6):1261–1273. doi: 10.1083/jcb.114.6.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givol D., Yayon A. Complexity of FGF receptors: genetic basis for structural diversity and functional specificity. FASEB J. 1992 Dec;6(15):3362–3369. [PubMed] [Google Scholar]
- Glinsmann-Gibson B. J., Korc M. Regulation of transforming growth factor-alpha mRNA expression in T3M4 human pancreatic carcinoma cells. Pancreas. 1991 Mar;6(2):142–149. doi: 10.1097/00006676-199103000-00003. [DOI] [PubMed] [Google Scholar]
- Gonzalez A. M., Buscaglia M., Fox R., Isacchi A., Sarmientos P., Farris J., Ong M., Martineau D., Lappi D. A., Baird A. Basic fibroblast growth factor in Dupuytren's contracture. Am J Pathol. 1992 Sep;141(3):661–671. [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Ferrara N., Schweigerer L., Neufeld G. Structural characterization and biological functions of fibroblast growth factor. Endocr Rev. 1987 May;8(2):95–114. doi: 10.1210/edrv-8-2-95. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Neufeld G., Schweigerer L. Molecular and biological characterization of fibroblast growth factor, an angiogenic factor which also controls the proliferation and differentiation of mesoderm and neuroectoderm derived cells. Cell Differ. 1986 Jul;19(1):1–17. doi: 10.1016/0045-6039(86)90021-7. [DOI] [PubMed] [Google Scholar]
- Jaye M., Howk R., Burgess W., Ricca G. A., Chiu I. M., Ravera M. W., O'Brien S. J., Modi W. S., Maciag T., Drohan W. N. Human endothelial cell growth factor: cloning, nucleotide sequence, and chromosome localization. Science. 1986 Aug 1;233(4763):541–545. doi: 10.1126/science.3523756. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M. The fibroblast growth factor family: structural and biological properties. Prog Growth Factor Res. 1989;1(4):207–235. doi: 10.1016/0955-2235(89)90012-4. [DOI] [PubMed] [Google Scholar]
- Korc M., Chandrasekar B., Yamanaka Y., Friess H., Buchier M., Beger H. G. Overexpression of the epidermal growth factor receptor in human pancreatic cancer is associated with concomitant increases in the levels of epidermal growth factor and transforming growth factor alpha. J Clin Invest. 1992 Oct;90(4):1352–1360. doi: 10.1172/JCI116001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korc M., Owerbach D., Quinto C., Rutter W. J. Pancreatic islet-acinar cell interaction: amylase messenger RNA levels ar determined by insulin. Science. 1981 Jul 17;213(4505):351–353. doi: 10.1126/science.6166044. [DOI] [PubMed] [Google Scholar]
- Li S., Shipley G. D. Expression of multiple species of basic fibroblast growth factor mRNA and protein in normal and tumor-derived mammary epithelial cells in culture. Cell Growth Differ. 1991 Apr;2(4):195–202. [PubMed] [Google Scholar]
- Massagué J., Cheifetz S., Laiho M., Ralph D. A., Weis F. M., Zentella A. Transforming growth factor-beta. Cancer Surv. 1992;12:81–103. [PubMed] [Google Scholar]
- Massagué J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- McNeil P. L., Muthukrishnan L., Warder E., D'Amore P. A. Growth factors are released by mechanically wounded endothelial cells. J Cell Biol. 1989 Aug;109(2):811–822. doi: 10.1083/jcb.109.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignatti P., Morimoto T., Rifkin D. B. Basic fibroblast growth factor released by single, isolated cells stimulates their migration in an autocrine manner. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11007–11011. doi: 10.1073/pnas.88.24.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H., Maki M., Oishi K., Jaye M., Igarashi K., Yoshida O., Hatanaka M. Increased expression of genes for basic fibroblast growth factor and transforming growth factor type beta 2 in human benign prostatic hyperplasia. Prostate. 1990;16(1):71–80. doi: 10.1002/pros.2990160108. [DOI] [PubMed] [Google Scholar]
- Moscatelli D., Presta M., Rifkin D. B. Purification of a factor from human placenta that stimulates capillary endothelial cell protease production, DNA synthesis, and migration. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2091–2095. doi: 10.1073/pnas.83.7.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell P. P., Klagsbrun M. Three forms of rat basic fibroblast growth factor are made from a single mRNA and localize to the nucleus. J Cell Physiol. 1991 Aug;148(2):202–210. doi: 10.1002/jcp.1041480204. [DOI] [PubMed] [Google Scholar]
- Prats A. C., Vagner S., Prats H., Amalric F. cis-acting elements involved in the alternative translation initiation process of human basic fibroblast growth factor mRNA. Mol Cell Biol. 1992 Oct;12(10):4796–4805. doi: 10.1128/mcb.12.10.4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapraeger A. C., Krufka A., Olwin B. B. Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science. 1991 Jun 21;252(5013):1705–1708. doi: 10.1126/science.1646484. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Sporn M. B., Assoian R. K., Smith J. M., Roche N. S., Wakefield L. M., Heine U. I., Liotta L. A., Falanga V., Kehrl J. H. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksela O., Moscatelli D., Rifkin D. B. The opposing effects of basic fibroblast growth factor and transforming growth factor beta on the regulation of plasminogen activator activity in capillary endothelial cells. J Cell Biol. 1987 Aug;105(2):957–963. doi: 10.1083/jcb.105.2.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarles H., Bernard J. P., Johnson C. Pathogenesis and epidemiology of chronic pancreatitis. Annu Rev Med. 1989;40:453–468. doi: 10.1146/annurev.me.40.020189.002321. [DOI] [PubMed] [Google Scholar]
- Schulze-Osthoff K., Risau W., Vollmer E., Sorg C. In situ detection of basic fibroblast growth factor by highly specific antibodies. Am J Pathol. 1990 Jul;137(1):85–92. [PMC free article] [PubMed] [Google Scholar]
- Shimoyama Y., Gotoh M., Ino Y., Sakamoto M., Kato K., Hirohashi S. Characterization of high-molecular-mass forms of basic fibroblast growth factor produced by hepatocellular carcinoma cells: possible involvement of basic fibroblast growth factor in hepatocarcinogenesis. Jpn J Cancer Res. 1991 Nov;82(11):1263–1270. doi: 10.1111/j.1349-7006.1991.tb01791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Transforming growth factor-beta: recent progress and new challenges. J Cell Biol. 1992 Dec;119(5):1017–1021. doi: 10.1083/jcb.119.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox J. N., Smith K. M., Williams L. T., Schwartz S. M., Gordon D. Platelet-derived growth factor mRNA detection in human atherosclerotic plaques by in situ hybridization. J Clin Invest. 1988 Sep;82(3):1134–1143. doi: 10.1172/JCI113671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka Y., Friess H., Buchler M., Beger H. G., Uchida E., Onda M., Kobrin M. S., Korc M. Overexpression of acidic and basic fibroblast growth factors in human pancreatic cancer correlates with advanced tumor stage. Cancer Res. 1993 Nov 1;53(21):5289–5296. [PubMed] [Google Scholar]
- Yamanaka Y., Friess H., Büchler M., Beger H. G., Gold L. I., Korc M. Synthesis and expression of transforming growth factor beta-1, beta-2, and beta-3 in the endocrine and exocrine pancreas. Diabetes. 1993 May;42(5):746–756. doi: 10.2337/diab.42.5.746. [DOI] [PubMed] [Google Scholar]
- Yayon A., Klagsbrun M., Esko J. D., Leder P., Ornitz D. M. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991 Feb 22;64(4):841–848. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]