Abstract
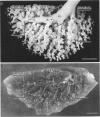
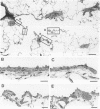
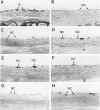
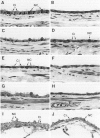
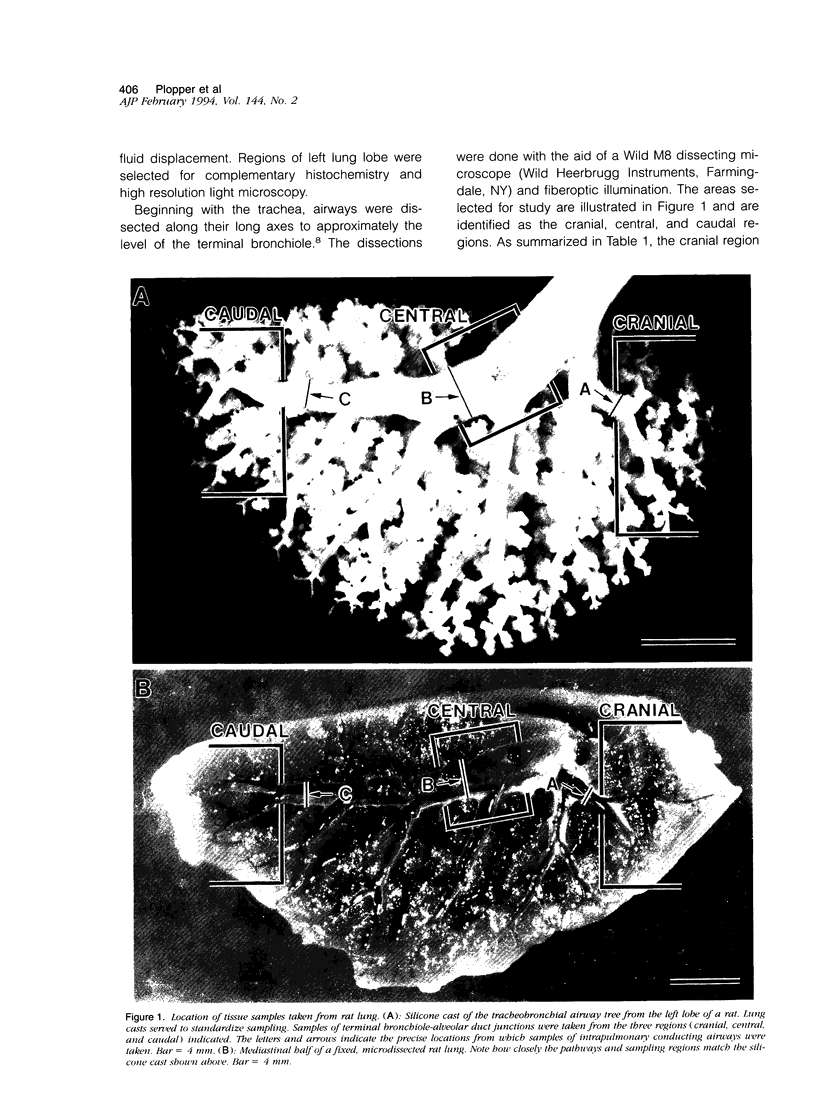
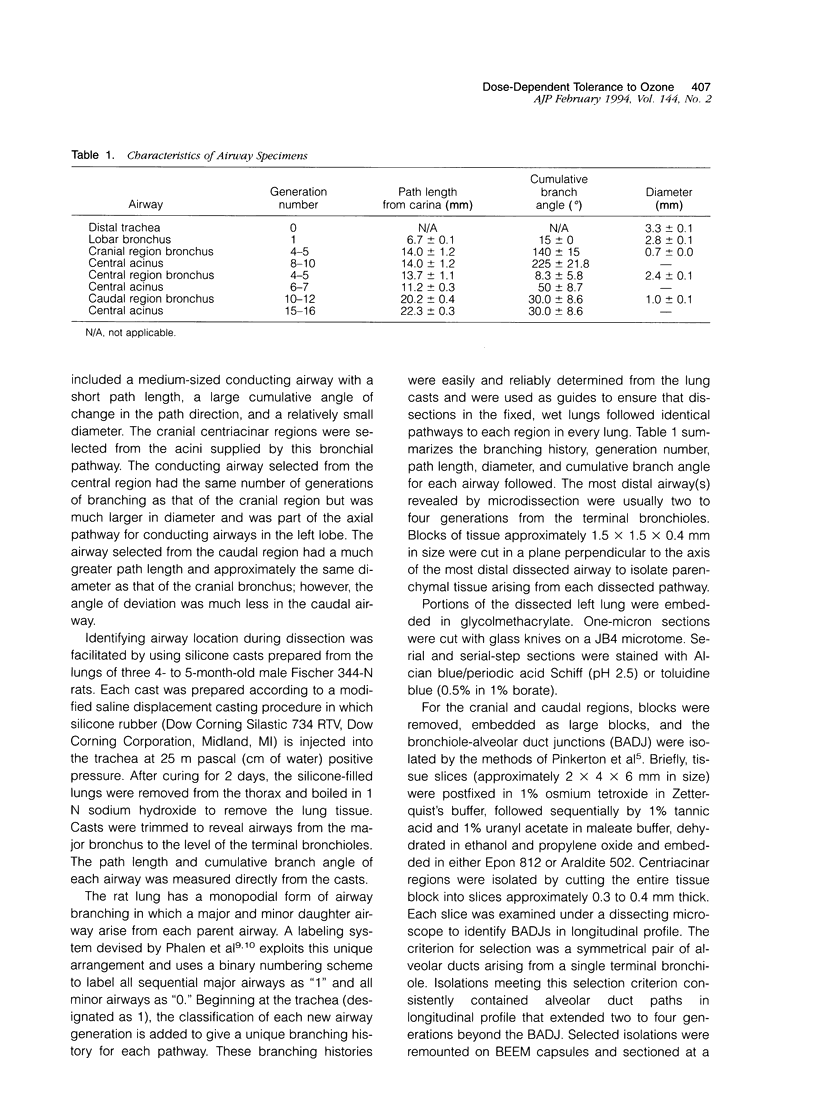
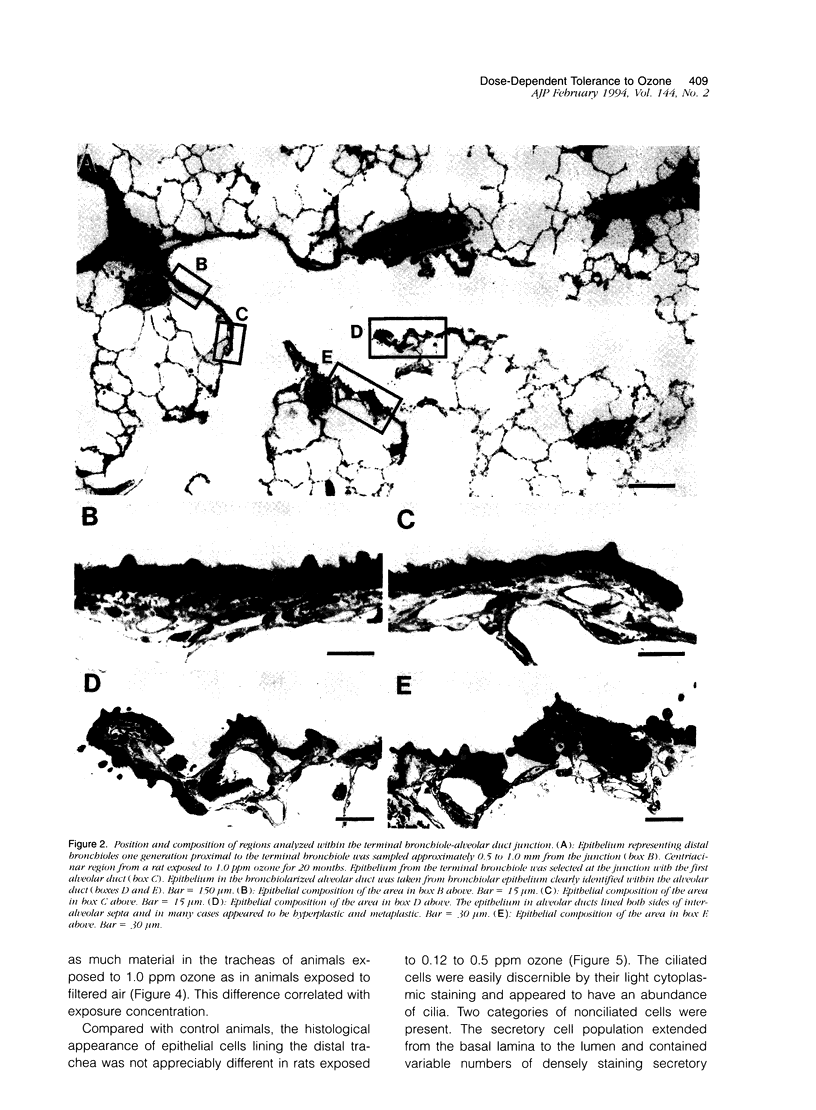
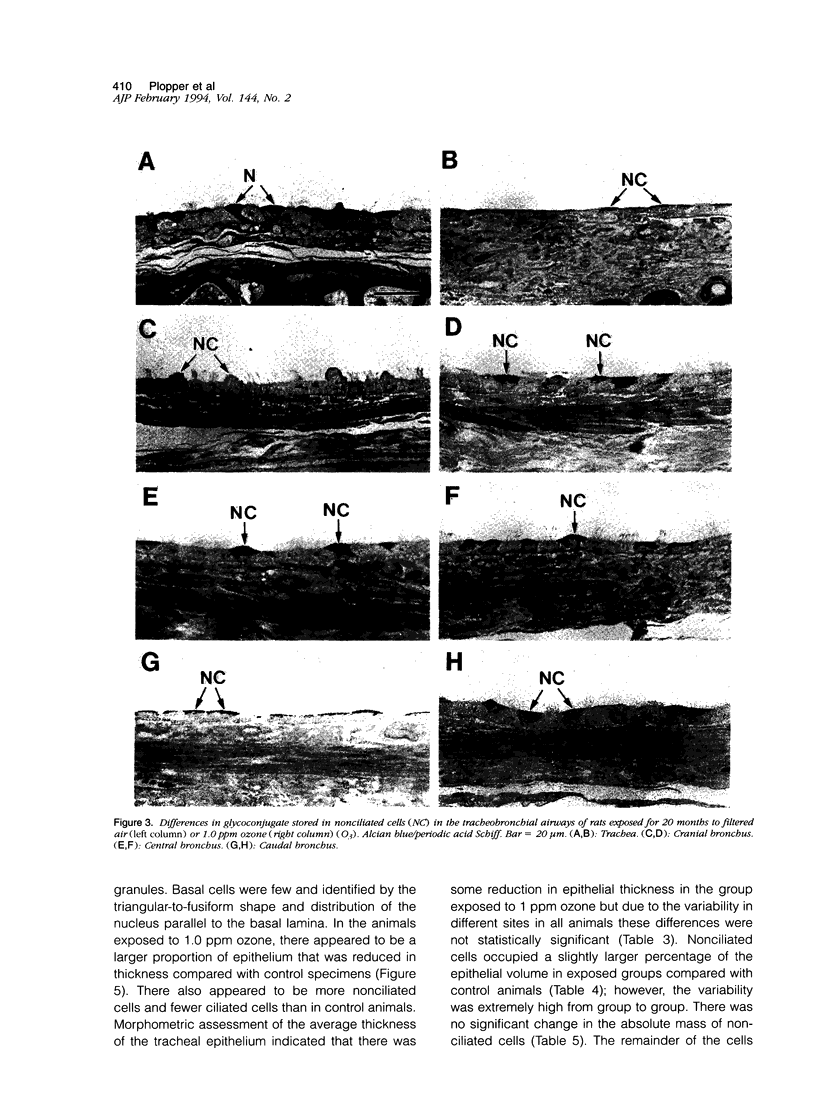
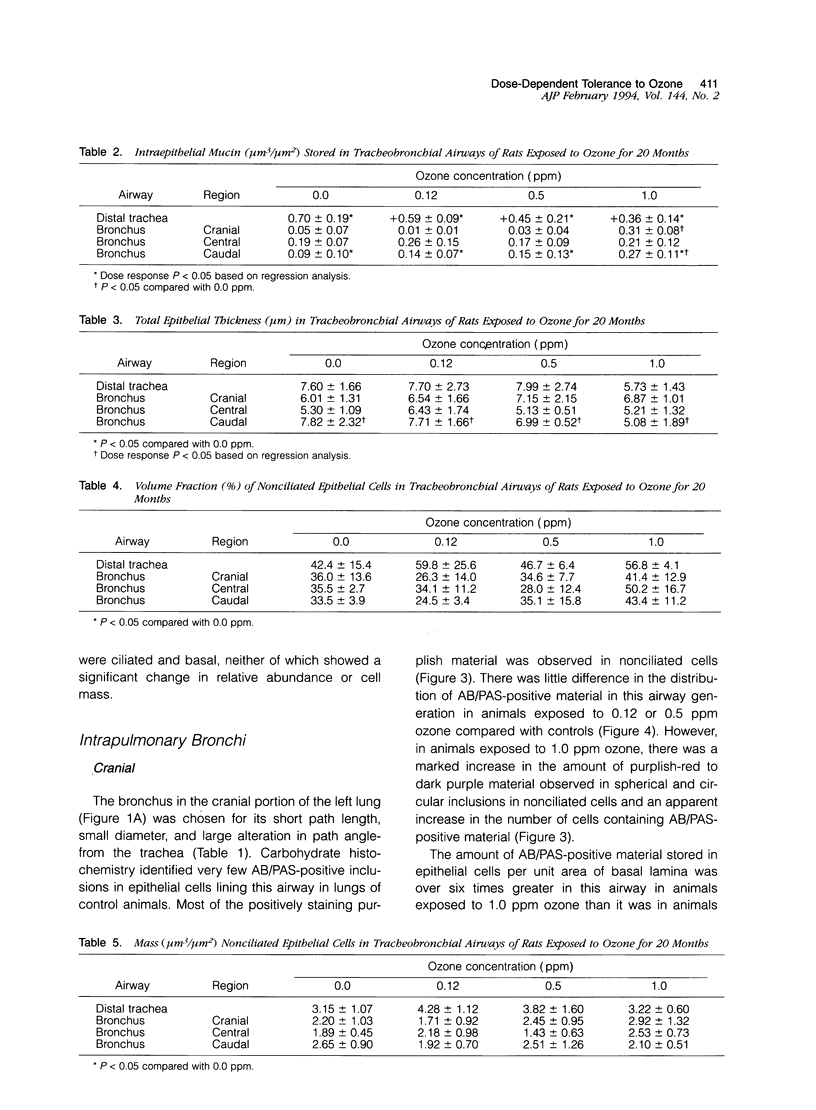
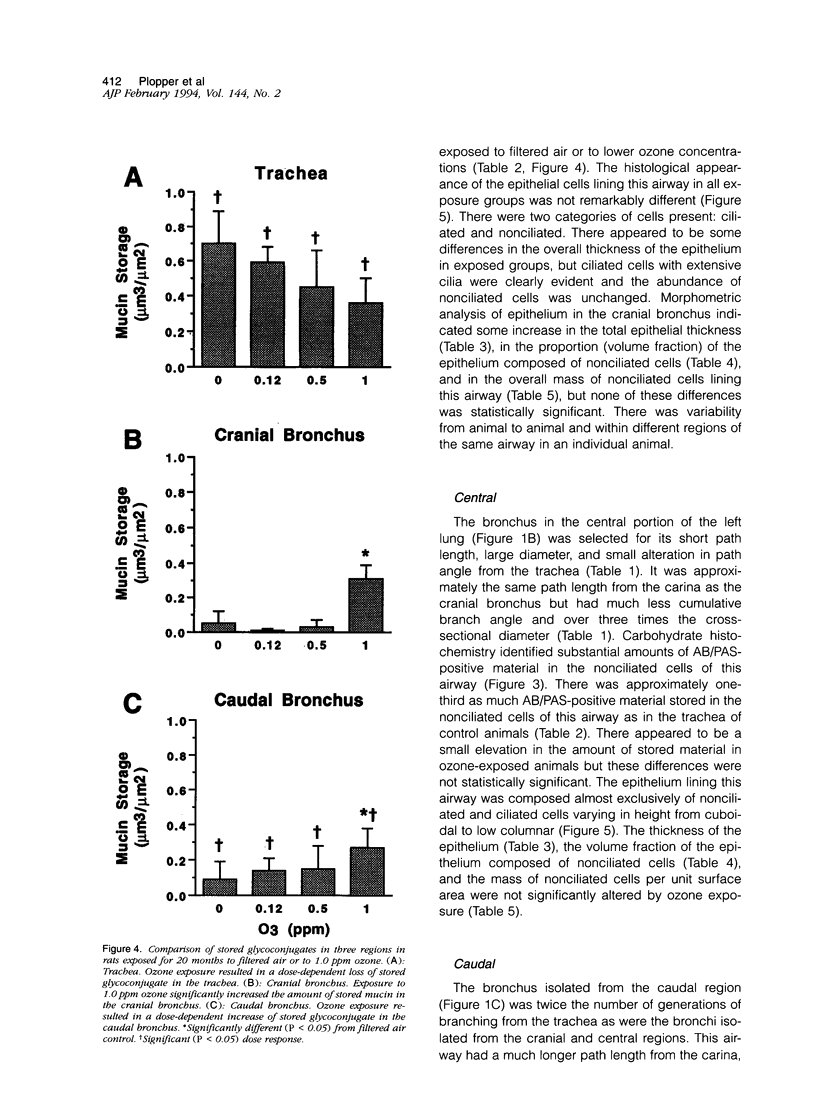
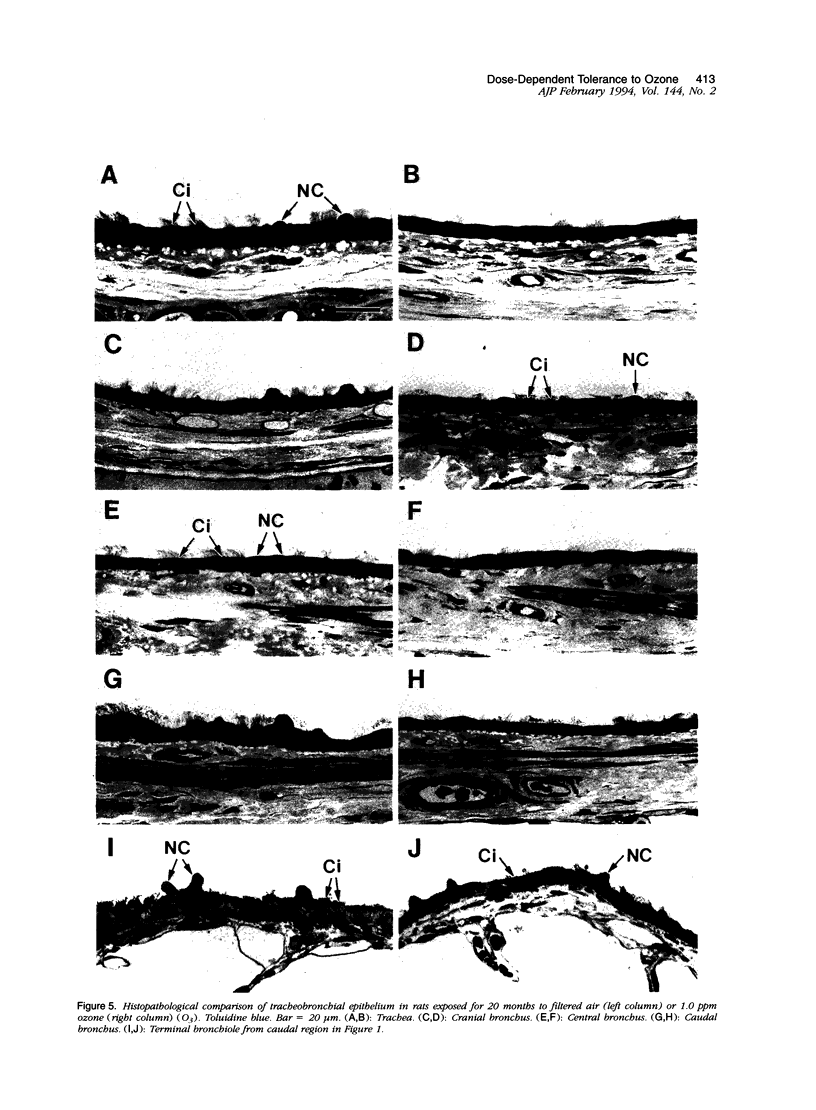
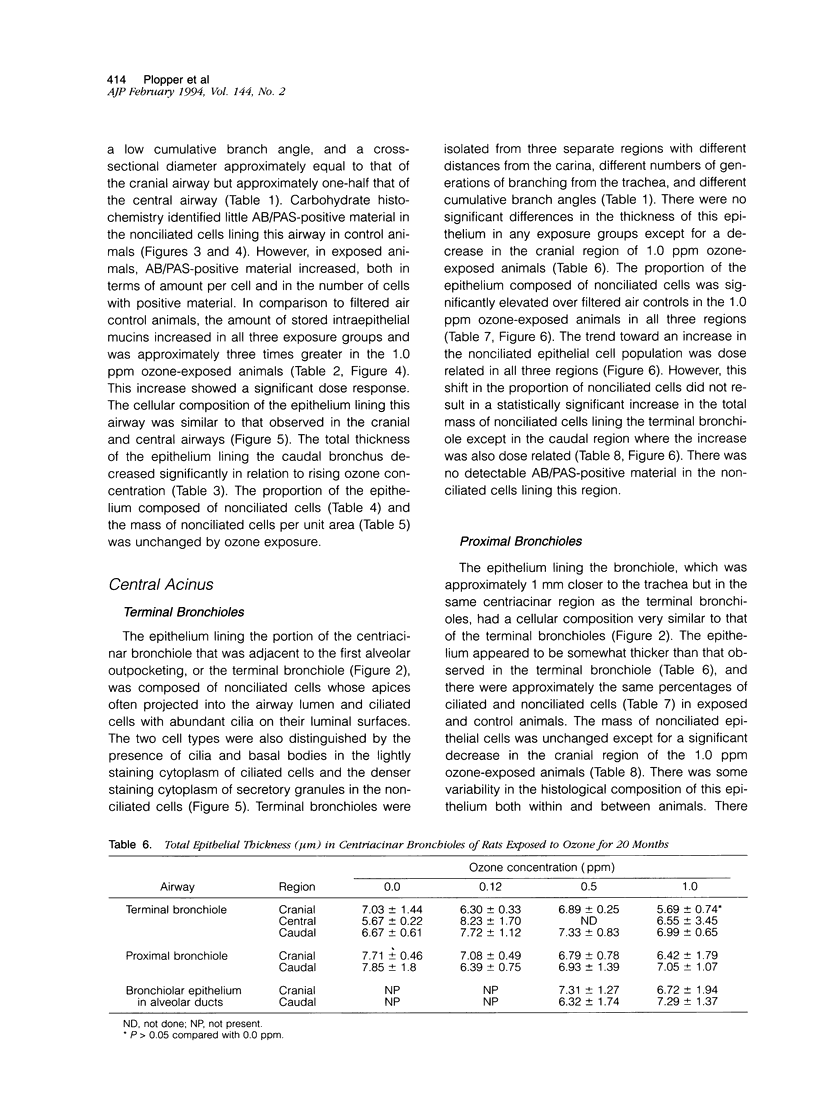
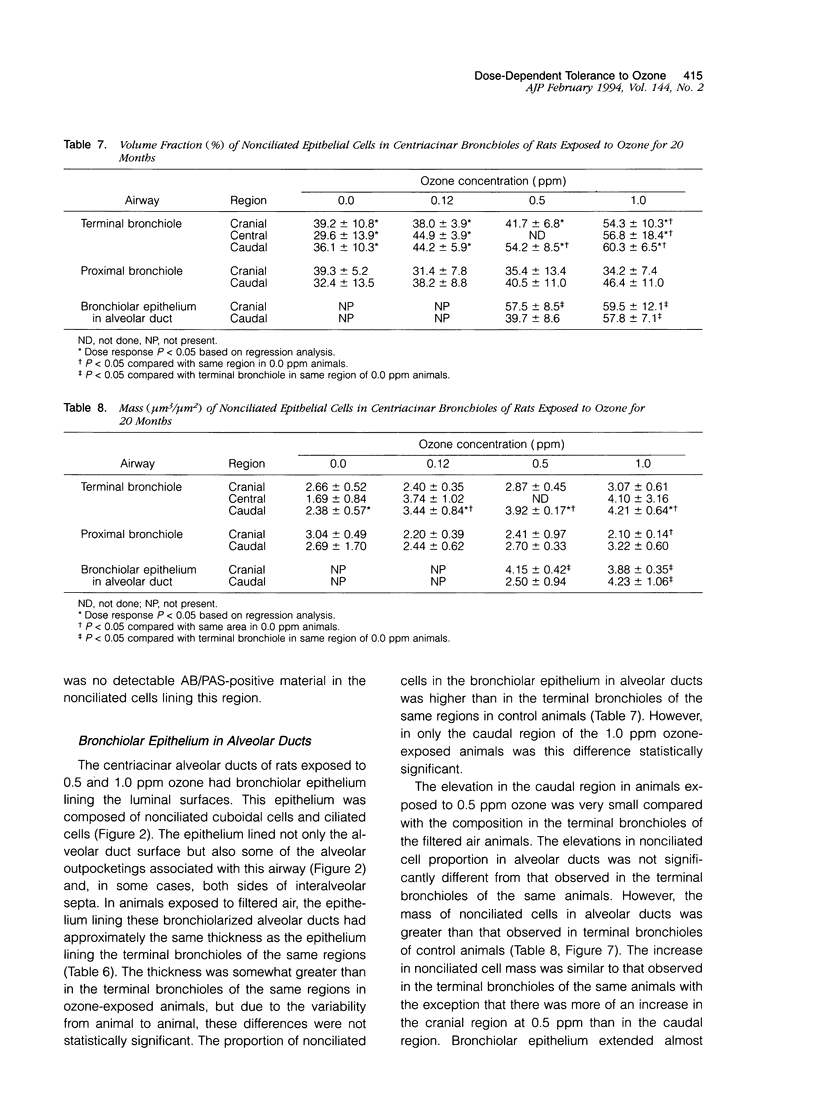
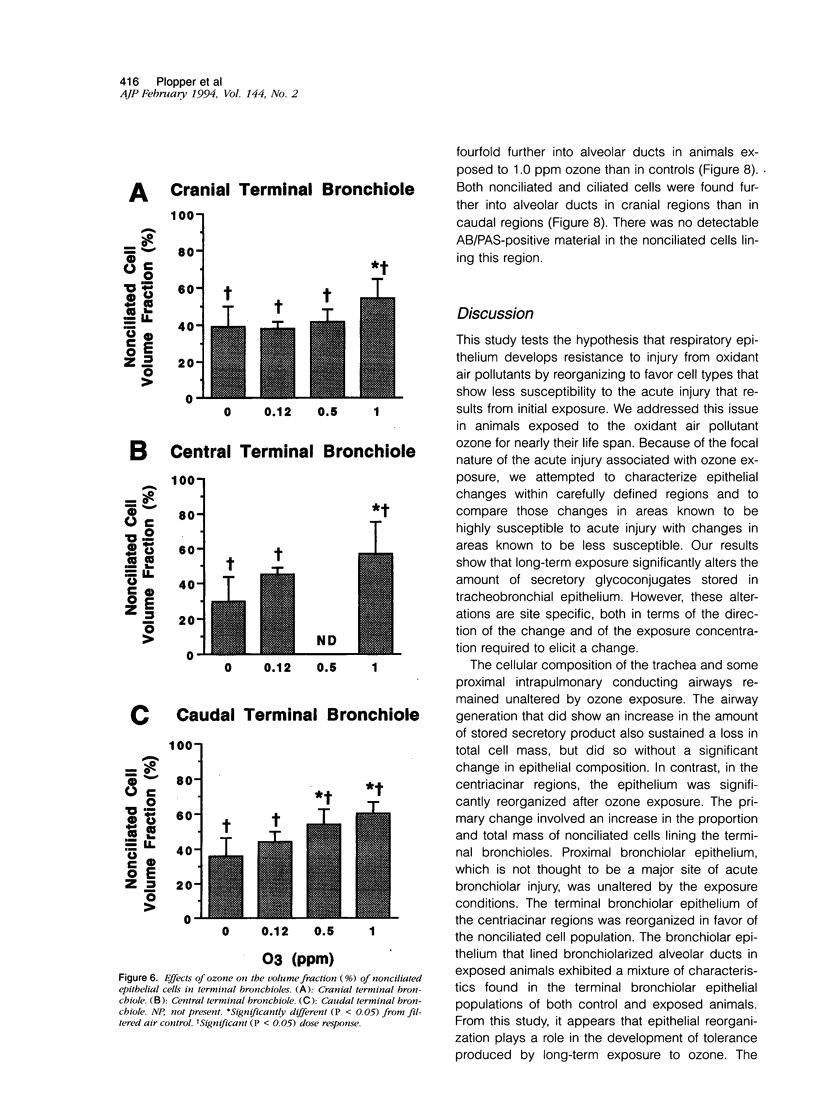
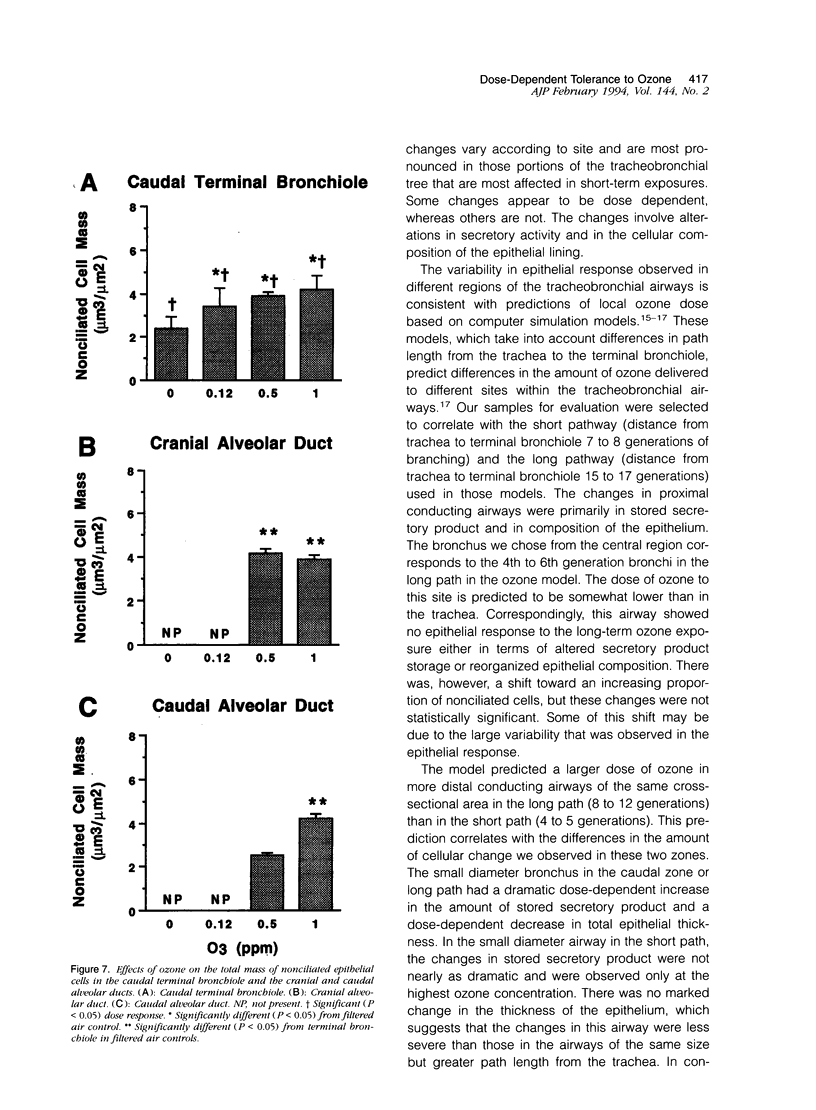
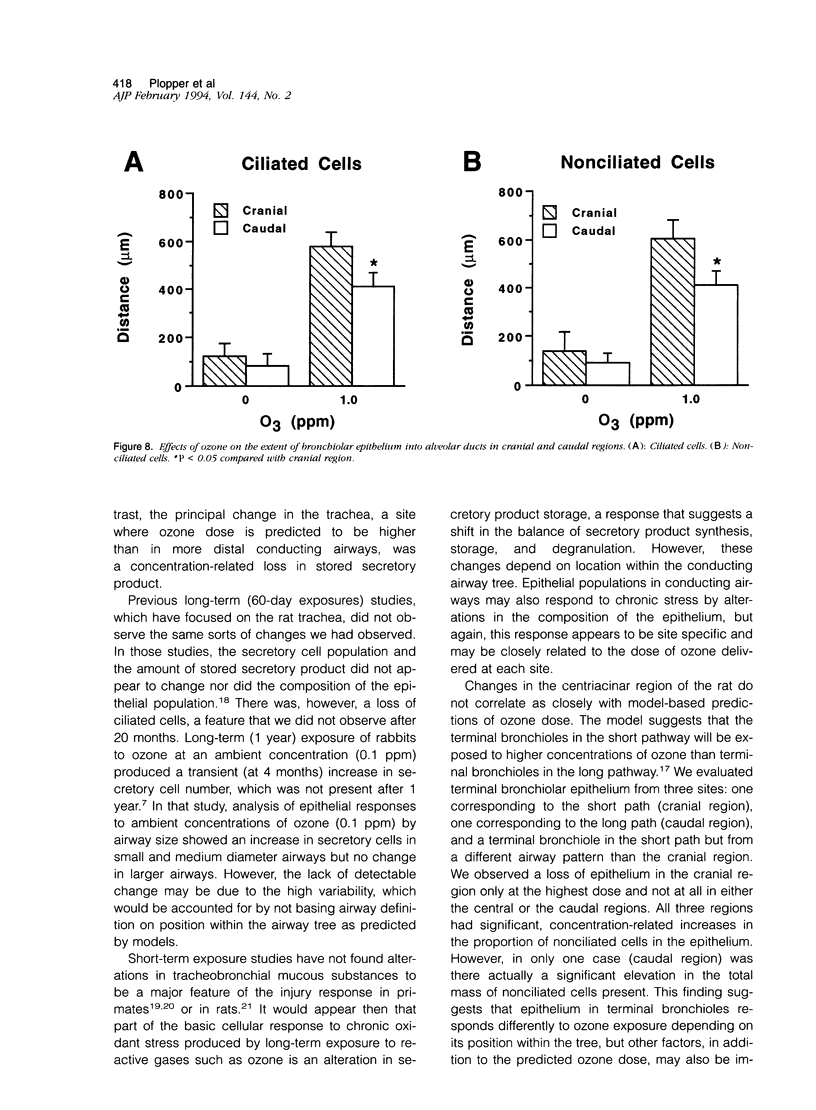
Two salient features of the pulmonary response to reactive oxidant air pollutants such as ozone are the heterogeneity of response by site within the respiratory tract and the development of tolerance to injury with repeated exposure. The purpose of this study was to establish whether the development of tolerance to long-term exposure is associated with changes in the tracheobronchial epithelium. Male F344-N rats were exposed to 0, 0.12, 0.5, or 1.0 ppm ozone 6 hours/day for 5 days/week for 20 months and killed 1 week post-exposure. Samples for light microscopic morphometry were obtained by microdissection from each infusion-fixed trachea and left lung lobe and included: 1) a cranial bronchus with short path length (generation no. 4 to 5) and small diameter; 2) a central bronchus with short path length (generation no. 4 to 5) and large diameter; and 3) a caudal bronchus with long path length (generation no. 10 to 12) and small diameter. In addition, three sites within the central acinus were examined from cranial, central, and caudal regions. These sites included terminal bronchiole, 0.5 to 1 mm proximal to terminal bronchiole and bronchiolarized alveolar duct. Intraepithelial mucin storage (AB/PAS-positive material quantified by image analysis) within the trachea decreased with dose. Mucin storage was unchanged in the central bronchus, increased threefold with dose in the caudal bronchus, and was six times higher in the cranial bronchus at 1.0 ppm ozone. Epithelial composition was unchanged in trachea or any bronchi; however, we noted a significant dose-dependent increase in nonciliated cell mass and volume fraction in terminal bronchioles in all three regions. There was also a significant increase in nonciliated cell mass in the bronchiolarized alveolar ducts. Bronchiolar nonciliated cells were identified greater than fourfold further into alveolar ducts in 1.0 ppm exposed than in 0 ppm animals. Nonciliated cells occurred almost 200 microns deeper into alveolar ducts in cranial regions than in caudal regions. We conclude: 1) that the development of tolerance to injury produced by long-term exposure to ozone involves changes in airway epithelium and 2) that these changes are site specific and involve alterations in both secretory activity and cellular composition.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barr B. C., Hyde D. M., Plopper C. G., Dungworth D. L. A comparison of terminal airway remodeling in chronic daily versus episodic ozone exposure. Toxicol Appl Pharmacol. 1990 Dec;106(3):384–407. doi: 10.1016/0041-008x(90)90335-r. [DOI] [PubMed] [Google Scholar]
- Boorman G. A., Schwartz L. W., Dungworth D. L. Pulmonary effects of prolonged ozone insult in rats. Morphometric evaluation of the central acinus. Lab Invest. 1980 Aug;43(2):108–115. [PubMed] [Google Scholar]
- Evans M. J., Shami S. G., Cabral-Anderson L. J., Dekker N. P. Role of nonciliated cells in renewal of the bronchial epithelium of rats exposed to NO2. Am J Pathol. 1986 Apr;123(1):126–133. [PMC free article] [PubMed] [Google Scholar]
- Hyde D. M., Hubbard W. C., Wong V., Wu R., Pinkerton K., Plopper C. G. Ozone-induced acute tracheobronchial epithelial injury: relationship to granulocyte emigration in the lung. Am J Respir Cell Mol Biol. 1992 May;6(5):481–497. doi: 10.1165/ajrcmb/6.5.481. [DOI] [PubMed] [Google Scholar]
- Hyde D. M., Magliano D. J., Plopper C. G. Morphometric assessment of pulmonary toxicity in the rodent lung. Toxicol Pathol. 1991;19(4 Pt 1):428–446. doi: 10.1177/0192623391019004-112. [DOI] [PubMed] [Google Scholar]
- Ionescu J., Marinescu D., Tapu V., Eskenasy A. Experimental chronic obstructive lung disease. I. Bronchopulmonary changes induced in rabbits by prolonged exposure to formaldehyde. Morphol Embryol (Bucur) 1978 Jul-Sep;24(3):233–242. [PubMed] [Google Scholar]
- Lamb D., Reid L. Goblet cell increase in rat bronchial epithelium after exposure to cigarette and cigar tobacco smoke. Br Med J. 1969 Jan 4;1(5635):33–35. doi: 10.1136/bmj.1.5635.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb D., Reid L. Mitotic rates, goblet cell increase and histochemical changes in mucus in rat bronchial epithelium during exposure to sulphur dioxide. J Pathol Bacteriol. 1968 Jul;96(1):97–111. doi: 10.1002/path.1700960111. [DOI] [PubMed] [Google Scholar]
- Mellick P. W., Dungworth D. L., Schwartz L. W., Tyler W. S. Short term morphologic effects of high ambient levels of ozone on lungs of rhesus monkeys. Lab Invest. 1977 Jan;36(1):82–90. [PubMed] [Google Scholar]
- Mercer R. R., Crapo J. D. Three-dimensional reconstruction of the rat acinus. J Appl Physiol (1985) 1987 Aug;63(2):785–794. doi: 10.1152/jappl.1987.63.2.785. [DOI] [PubMed] [Google Scholar]
- Nikula K. J., Wilson D. W., Giri S. N., Plopper C. G., Dungworth D. L. The response of the rat tracheal epithelium to ozone exposure. Injury, adaptation, and repair. Am J Pathol. 1988 May;131(2):373–384. [PMC free article] [PubMed] [Google Scholar]
- Overton J. H., Graham R. C., Miller F. J. A model of the regional uptake of gaseous pollutants in the lung. II. The sensitivity of ozone uptake in laboratory animal lungs to anatomical and ventilatory parameters. Toxicol Appl Pharmacol. 1987 May;88(3):418–432. doi: 10.1016/0041-008x(87)90216-x. [DOI] [PubMed] [Google Scholar]
- Penha P. D., Werthamer S. Pulmonary lesions induced by long-term exposure to ozone. II. Ultrastructure observations of proliferative and regressive lesions. Arch Environ Health. 1974 Nov;29(5):282–289. doi: 10.1080/00039896.1974.10666588. [DOI] [PubMed] [Google Scholar]
- Phalen R. F., Yeh H. C., Schum G. M., Raabe O. G. Application of an idealized model to morphometry of the mammalian tracheobronchial tree. Anat Rec. 1978 Feb;190(2):167–176. doi: 10.1002/ar.1091900202. [DOI] [PubMed] [Google Scholar]
- Pinkerton K. E., Dodge D. E., Cederdahl-Demmler J., Wong V. J., Peake J., Haselton C. J., Mellick P. W., Singh G., Plopper C. G. Differentiated bronchiolar epithelium in alveolar ducts of rats exposed to ozone for 20 months. Am J Pathol. 1993 Mar;142(3):947–956. [PMC free article] [PubMed] [Google Scholar]
- Plopper C. G., Macklin J., Nishio S. J., Hyde D. M., Buckpitt A. R. Relationship of cytochrome P-450 activity to Clara cell cytotoxicity. III. Morphometric comparison of changes in the epithelial populations of terminal bronchioles and lobar bronchi in mice, hamsters, and rats after parenteral administration of naphthalene. Lab Invest. 1992 Nov;67(5):553–565. [PubMed] [Google Scholar]
- Schlesinger R. B., Gorczynski J. E., Dennison J., Richards L., Kinney P. L., Bosland M. C. Long-term intermittent exposure to sulfuric acid aerosol, ozone, and their combination: alterations in tracheobronchial mucociliary clearance and epithelial secretory cells. Exp Lung Res. 1992 Jul-Aug;18(4):505–534. doi: 10.3109/01902149209064343. [DOI] [PubMed] [Google Scholar]
- Schwartz L. W., Dungworth D. L., Mustafa M. G., Tarkington B. K., Tyler W. S. Pulmonary responses of rats to ambient levels of ozone: effects of 7-day intermittent or continuous exposure. Lab Invest. 1976 Jun;34(6):565–578. [PubMed] [Google Scholar]
- Spicer S. S., Chakrin L. W., Wardell J. R., Jr Effect of chronic sulfur dioxide inhalation on the carbohydrate histochemistry and histology of the canine respiratory tract. Am Rev Respir Dis. 1974 Jul;110(1):13–24. doi: 10.1164/arrd.1974.110.6P2.13. [DOI] [PubMed] [Google Scholar]
- Wilson D. W., Plopper C. G., Dungworth D. L. The response of the macaque tracheobronchial epithelium to acute ozone injury. A quantitative ultrastructural and autoradiographic study. Am J Pathol. 1984 Aug;116(2):193–206. [PMC free article] [PubMed] [Google Scholar]