Abstract
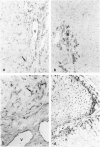
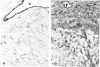
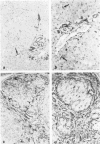
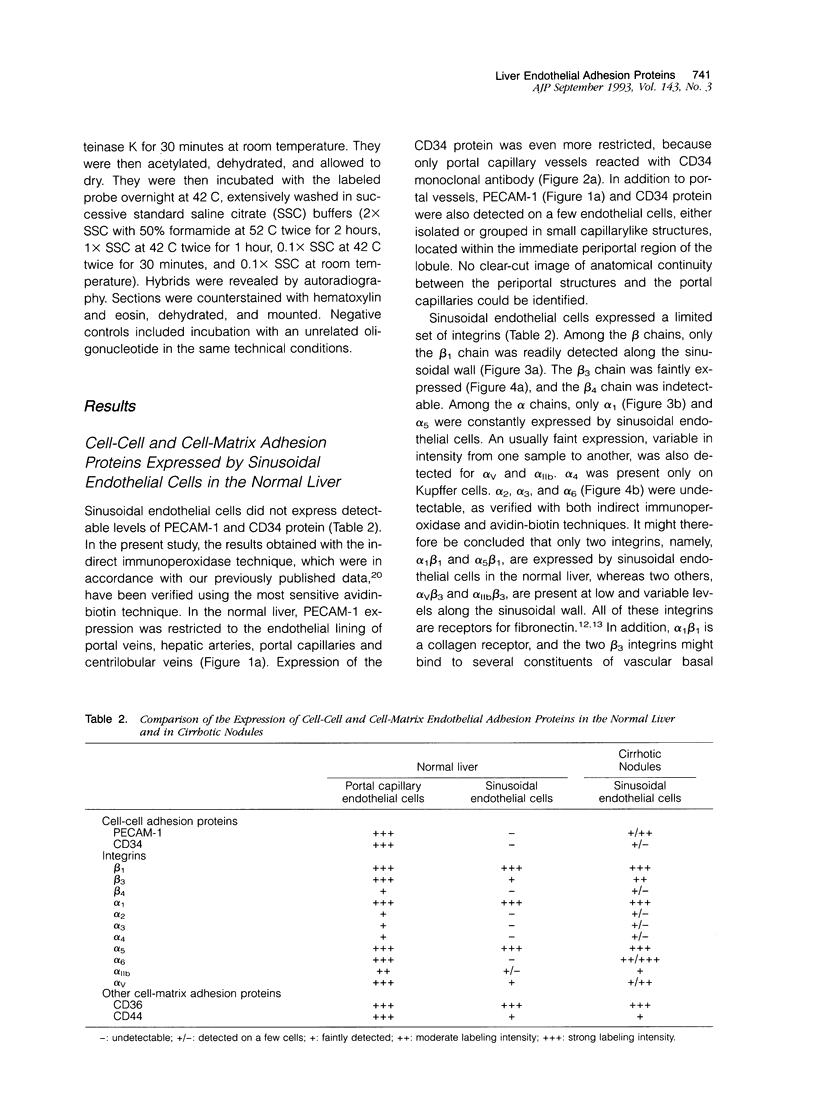
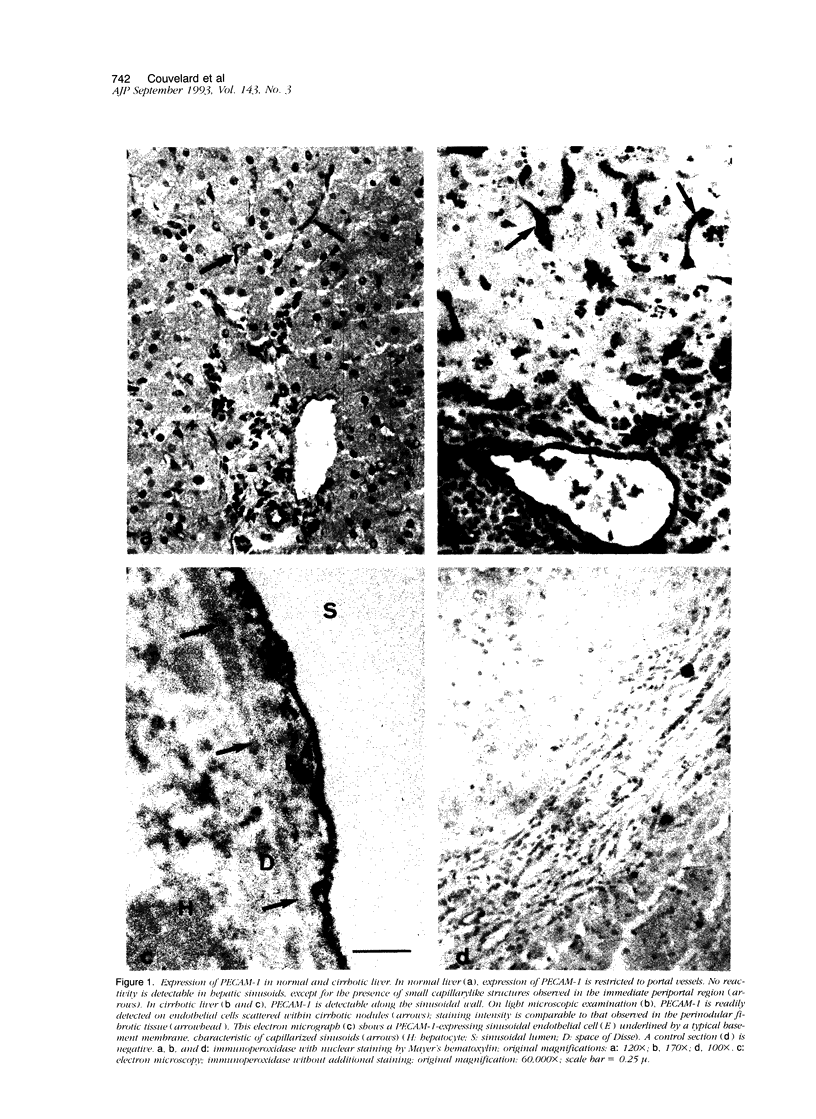
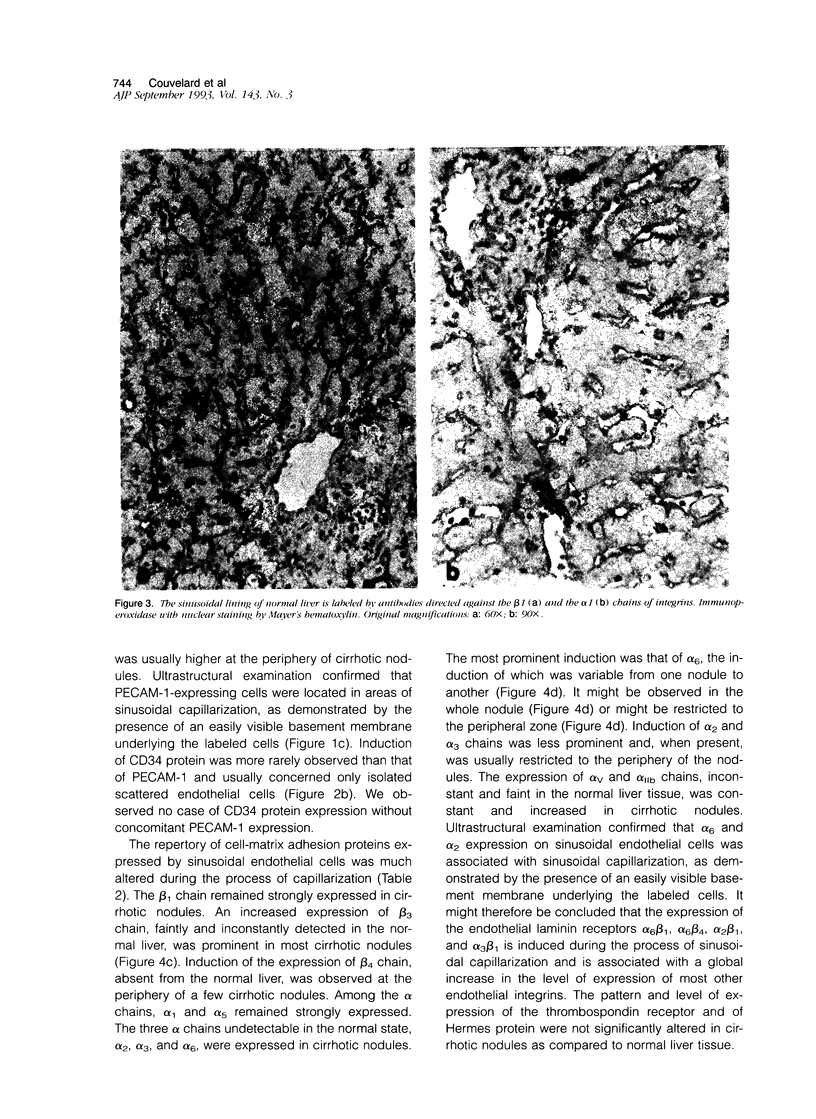
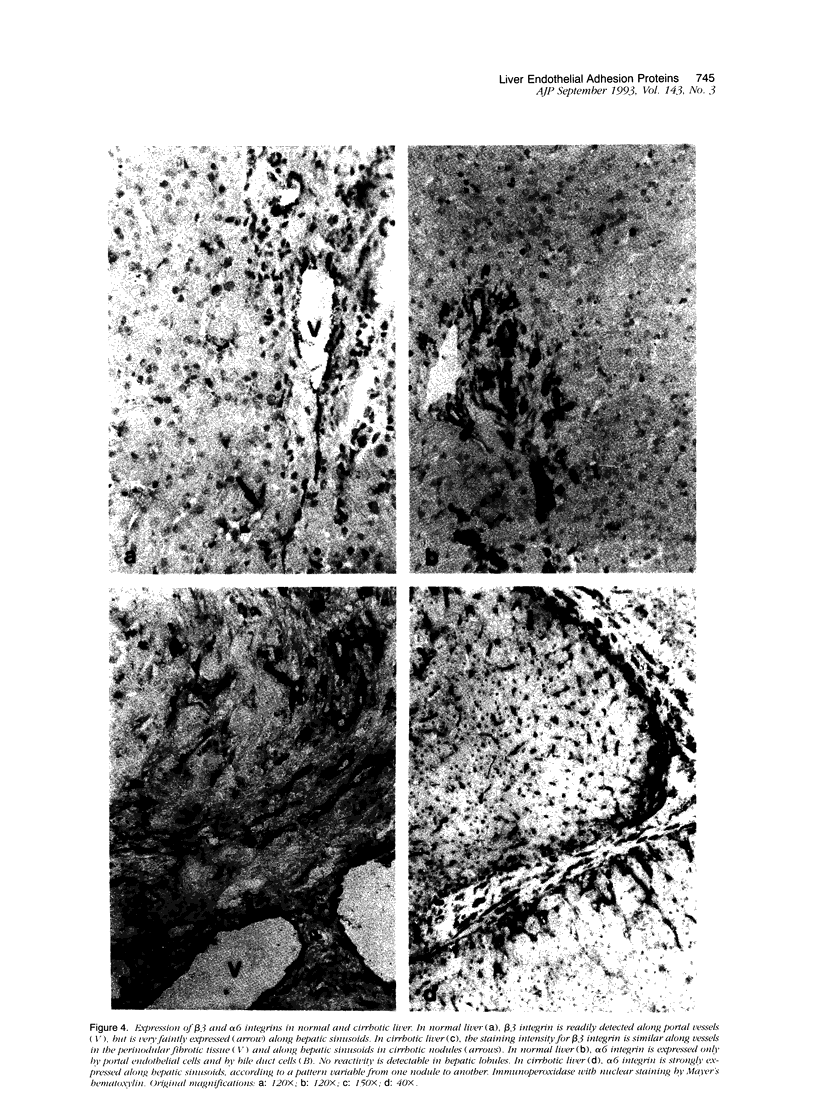
We compared the expression of cell-cell and cell-matrix adhesion proteins by sinusoidal endothelial cells in normal human liver, in which the endothelial lining of hepatic sinusoids is discontinuous and devoid of basement membrane, and in cirrhosis, during which sinusoids might undergo a process of capillarization and acquire a continuous lining and a typical basement membrane. In normal liver, sinusoidal endothelial cells displayed a very restricted repertory of cell-adhesion molecules: the intercellular adhesion molecules PECAM-1 and CD34 were undetectable and only two integrins, alpha 1 beta 1 and alpha 5 beta 1, were present, whereas the laminin receptors alpha 6 beta 1 and alpha 2 beta 1 were undetectable and the beta 3 integrins were faintly expressed. In capillarized sinusoids, sinusoidal endothelial cells displayed striking changes in their repertory of cell-adhesion molecules, including the expression of PECAM-1 protein and messenger RNAs and the induction of the laminin receptors alpha 6 beta 1 and alpha 2 beta 1. Such changes co-localized with subendothelial laminin deposits. In conclusion, normal sinusoidal endothelial cells express a distinctive set of cell-adhesion molecules, adapted to their structural and microenvironmental characteristics, and this repertory is dramatically modified during sinusoidal capillarization, possibly as a consequence of the concomitant matrix changes.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M., Muller W. A., Buck C. A., Newman P. J. Molecular and cellular properties of PECAM-1 (endoCAM/CD31): a novel vascular cell-cell adhesion molecule. J Cell Biol. 1991 Sep;114(5):1059–1068. doi: 10.1083/jcb.114.5.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albelda S. M., Oliver P. D., Romer L. H., Buck C. A. EndoCAM: a novel endothelial cell-cell adhesion molecule. J Cell Biol. 1990 Apr;110(4):1227–1237. doi: 10.1083/jcb.110.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenson D. M., Bissell D. M. Glycosaminoglycan, proteoglycan, and hepatic fibrosis. Gastroenterology. 1987 Feb;92(2):536–538. doi: 10.1016/0016-5085(87)90155-7. [DOI] [PubMed] [Google Scholar]
- Aruffo A., Stamenkovic I., Melnick M., Underhill C. B., Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990 Jun 29;61(7):1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- Babbs C., Haboubi N. Y., Mellor J. M., Smith A., Rowan B. P., Warnes T. W. Endothelial cell transformation in primary biliary cirrhosis: a morphological and biochemical study. Hepatology. 1990 May;11(5):723–729. doi: 10.1002/hep.1840110503. [DOI] [PubMed] [Google Scholar]
- Biagini G., Ballardini G. Liver fibrosis and extracellular matrix. J Hepatol. 1989 Jan;8(1):115–124. doi: 10.1016/0168-8278(89)90170-0. [DOI] [PubMed] [Google Scholar]
- Bianchi F. B., Biagini G., Ballardini G., Cenacchi G., Faccani A., Pisi E., Laschi R., Liotta L., Garbisa S. Basement membrane production by hepatocytes in chronic liver disease. Hepatology. 1984 Nov-Dec;4(6):1167–1172. doi: 10.1002/hep.1840040612. [DOI] [PubMed] [Google Scholar]
- Bioulac-Sage P., Balabaud C. Proliferation and phenotypic expression of perisinusoidal cells. J Hepatol. 1992 Jul;15(3):284–287. doi: 10.1016/0168-8278(92)90057-v. [DOI] [PubMed] [Google Scholar]
- Brennan M. J., Oldberg A., Pierschbacher M. D., Ruoslahti E. Chondroitin/dermatan sulfate proteoglycan in human fetal membranes. Demonstration of an antigenically similar proteoglycan in fibroblasts. J Biol Chem. 1984 Nov 25;259(22):13742–13750. [PubMed] [Google Scholar]
- Cheresh D. A., Berliner S. A., Vicente V., Ruggeri Z. M. Recognition of distinct adhesive sites on fibrinogen by related integrins on platelets and endothelial cells. Cell. 1989 Sep 8;58(5):945–953. doi: 10.1016/0092-8674(89)90946-x. [DOI] [PubMed] [Google Scholar]
- Clément B., Rescan P. Y., Baffet G., Loréal O., Lehry D., Campion J. P., Guillouzo A. Hepatocytes may produce laminin in fibrotic liver and in primary culture. Hepatology. 1988 Jul-Aug;8(4):794–803. doi: 10.1002/hep.1840080417. [DOI] [PubMed] [Google Scholar]
- Fina L., Molgaard H. V., Robertson D., Bradley N. J., Monaghan P., Delia D., Sutherland D. R., Baker M. A., Greaves M. F. Expression of the CD34 gene in vascular endothelial cells. Blood. 1990 Jun 15;75(12):2417–2426. [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Grant D. S., Tashiro K., Segui-Real B., Yamada Y., Martin G. R., Kleinman H. K. Two different laminin domains mediate the differentiation of human endothelial cells into capillary-like structures in vitro. Cell. 1989 Sep 8;58(5):933–943. doi: 10.1016/0092-8674(89)90945-8. [DOI] [PubMed] [Google Scholar]
- Greenwalt D. E., Lipsky R. H., Ockenhouse C. F., Ikeda H., Tandon N. N., Jamieson G. A. Membrane glycoprotein CD36: a review of its roles in adherence, signal transduction, and transfusion medicine. Blood. 1992 Sep 1;80(5):1105–1115. [PubMed] [Google Scholar]
- Griffiths M. R., Keir S., Burt A. D. Basement membrane proteins in the space of Disse: a reappraisal. J Clin Pathol. 1991 Aug;44(8):646–648. doi: 10.1136/jcp.44.8.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn E., Wick G., Pencev D., Timpl R. Distribution of basement membrane proteins in normal and fibrotic human liver: collagen type IV, laminin, and fibronectin. Gut. 1980 Jan;21(1):63–71. doi: 10.1136/gut.21.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimark R. L., Degner M., Schwartz S. M. Identification of a Ca2(+)-dependent cell-cell adhesion molecule in endothelial cells. J Cell Biol. 1990 May;110(5):1745–1756. doi: 10.1083/jcb.110.5.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Ingber D. E., Folkman J. How does extracellular matrix control capillary morphogenesis? Cell. 1989 Sep 8;58(5):803–805. doi: 10.1016/0092-8674(89)90928-8. [DOI] [PubMed] [Google Scholar]
- Inuzuka S., Ueno T., Torimura T., Tamaki S., Sakata R., Sata M., Yoshida H., Tanikawa K. Vitronectin in liver disorders: biochemical and immunohistochemical studies. Hepatology. 1992 Apr;15(4):629–636. doi: 10.1002/hep.1840150413. [DOI] [PubMed] [Google Scholar]
- Kirchhofer D., Languino L. R., Ruoslahti E., Pierschbacher M. D. Alpha 2 beta 1 integrins from different cell types show different binding specificities. J Biol Chem. 1990 Jan 15;265(2):615–618. [PubMed] [Google Scholar]
- Kramer R. H., Cheng Y. F., Clyman R. Human microvascular endothelial cells use beta 1 and beta 3 integrin receptor complexes to attach to laminin. J Cell Biol. 1990 Sep;111(3):1233–1243. doi: 10.1083/jcb.111.3.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y., Kleinman H. K., Martin G. R., Lawley T. J. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J Cell Biol. 1988 Oct;107(4):1589–1598. doi: 10.1083/jcb.107.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Languino L. R., Gehlsen K. R., Wayner E., Carter W. G., Engvall E., Ruoslahti E. Endothelial cells use alpha 2 beta 1 integrin as a laminin receptor. J Cell Biol. 1989 Nov;109(5):2455–2462. doi: 10.1083/jcb.109.5.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E. C., Lotz M. M., Steele G. D., Jr, Mercurio A. M. The integrin alpha 6 beta 4 is a laminin receptor. J Cell Biol. 1992 May;117(3):671–678. doi: 10.1083/jcb.117.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loréal O., Clément B., Schuppan D., Rescan P. Y., Rissel M., Guillouzo A. Distribution and cellular origin of collagen VI during development and in cirrhosis. Gastroenterology. 1992 Mar;102(3):980–987. doi: 10.1016/0016-5085(92)90186-3. [DOI] [PubMed] [Google Scholar]
- Mak K. M., Lieber C. S. Lipocytes and transitional cells in alcoholic liver disease: a morphometric study. Hepatology. 1988 Sep-Oct;8(5):1027–1033. doi: 10.1002/hep.1840080508. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A., Martinez J. The role of capillarization in hepatic failure: studies in carbon tetrachloride-induced cirrhosis. Hepatology. 1991 Nov;14(5):864–874. doi: 10.1002/hep.1840140519. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A. The hepatic extracellular matrix. I. Electron immunohistochemical studies in normal rat liver. Lab Invest. 1984 Jul;51(1):57–74. [PubMed] [Google Scholar]
- Martinez-Hernandez A. The hepatic extracellular matrix. II. Electron immunohistochemical studies in rats with CCl4-induced cirrhosis. Lab Invest. 1985 Aug;53(2):166–186. [PubMed] [Google Scholar]
- Maurice M., Durand-Schneider A. M., Garbarz M., Feldmann G. Characterization of rat hepatocyte plasma membrane domains by monoclonal antibodies. Eur J Cell Biol. 1985 Nov;39(1):122–129. [PubMed] [Google Scholar]
- McGuire R. F., Bissell D. M., Boyles J., Roll F. J. Role of extracellular matrix in regulating fenestrations of sinusoidal endothelial cells isolated from normal rat liver. Hepatology. 1992 Jun;15(6):989–997. doi: 10.1002/hep.1840150603. [DOI] [PubMed] [Google Scholar]
- Muller W. A., Ratti C. M., McDonnell S. L., Cohn Z. A. A human endothelial cell-restricted, externally disposed plasmalemmal protein enriched in intercellular junctions. J Exp Med. 1989 Aug 1;170(2):399–414. doi: 10.1084/jem.170.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Módis L., Martinez-Hernandez A. Hepatocytes modulate the hepatic microvascular phenotype. Lab Invest. 1991 Dec;65(6):661–670. [PubMed] [Google Scholar]
- Newman P. J., Berndt M. C., Gorski J., White G. C., 2nd, Lyman S., Paddock C., Muller W. A. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science. 1990 Mar 9;247(4947):1219–1222. doi: 10.1126/science.1690453. [DOI] [PubMed] [Google Scholar]
- Reid L. M., Fiorino A. S., Sigal S. H., Brill S., Holst P. A. Extracellular matrix gradients in the space of Disse: relevance to liver biology. Hepatology. 1992 Jun;15(6):1198–1203. doi: 10.1002/hep.1840150635. [DOI] [PubMed] [Google Scholar]
- Rieder H., Ramadori G., Meyer zum Büschenfelde K. H. Sinusoidal endothelial liver cells in vitro release endothelin--augmentation by transforming growth factor beta and Kupffer cell-conditioned media. Klin Wochenschr. 1991 Jun 18;69(9):387–391. doi: 10.1007/BF01647411. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Integrins. J Clin Invest. 1991 Jan;87(1):1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAFFNER F., POPER H. Capillarization of hepatic sinusoids in man. Gastroenterology. 1963 Mar;44:239–242. [PubMed] [Google Scholar]
- Schlingemann R. O., Rietveld F. J., de Waal R. M., Bradley N. J., Skene A. I., Davies A. J., Greaves M. F., Denekamp J., Ruiter D. J. Leukocyte antigen CD34 is expressed by a subset of cultured endothelial cells and on endothelial abluminal microprocesses in the tumor stroma. Lab Invest. 1990 Jun;62(6):690–696. [PubMed] [Google Scholar]
- Schuppan D. Structure of the extracellular matrix in normal and fibrotic liver: collagens and glycoproteins. Semin Liver Dis. 1990 Feb;10(1):1–10. doi: 10.1055/s-2008-1040452. [DOI] [PubMed] [Google Scholar]
- Scoazec J. Y., Feldmann G. Both macrophages and endothelial cells of the human hepatic sinusoid express the CD4 molecule, a receptor for the human immunodeficiency virus. Hepatology. 1990 Sep;12(3 Pt 1):505–510. doi: 10.1002/hep.1840120310. [DOI] [PubMed] [Google Scholar]
- Scoazec J. Y., Feldmann G. In situ immunophenotyping study of endothelial cells of the human hepatic sinusoid: results and functional implications. Hepatology. 1991 Nov;14(5):789–797. doi: 10.1002/hep.1840140508. [DOI] [PubMed] [Google Scholar]
- Simmons D. L., Walker C., Power C., Pigott R. Molecular cloning of CD31, a putative intercellular adhesion molecule closely related to carcinoembryonic antigen. J Exp Med. 1990 Jun 1;171(6):2147–2152. doi: 10.1084/jem.171.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier I., Bernuau D., Poliard A., Schoevaert D., Feldmann G. Detection of albumin mRNAs in rat liver by in situ hybridization: usefulness of paraffin embedding and comparison of various fixation procedures. J Histochem Cytochem. 1987 Apr;35(4):453–459. doi: 10.1177/35.4.3546490. [DOI] [PubMed] [Google Scholar]
- Van Eyken P., Sciot R., Desmet V. J. Expression of the novel extracellular matrix component tenascin in normal and diseased human liver. An immunohistochemical study. J Hepatol. 1990 Jul;11(1):43–52. doi: 10.1016/0168-8278(90)90270-2. [DOI] [PubMed] [Google Scholar]
- Volpes R., van den Oord J. J., Desmet V. J. Distribution of the VLA family of integrins in normal and pathological human liver tissue. Gastroenterology. 1991 Jul;101(1):200–206. doi: 10.1016/0016-5085(91)90478-4. [DOI] [PubMed] [Google Scholar]
- Wisse E., De Zanger R. B., Charels K., Van Der Smissen P., McCuskey R. S. The liver sieve: considerations concerning the structure and function of endothelial fenestrae, the sinusoidal wall and the space of Disse. Hepatology. 1985 Jul-Aug;5(4):683–692. doi: 10.1002/hep.1840050427. [DOI] [PubMed] [Google Scholar]
- Yamada S., Ichida T., Matsuda Y., Miyazaki Y., Hatano T., Hata K., Asakura H., Hirota N., Geerts A., Wisse E. Tenascin expression in human chronic liver disease and in hepatocellular carcinoma. Liver. 1992 Feb;12(1):10–16. doi: 10.1111/j.1600-0676.1992.tb00548.x. [DOI] [PubMed] [Google Scholar]