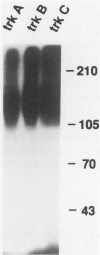
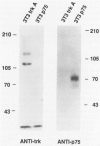
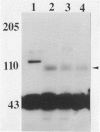
Abstract
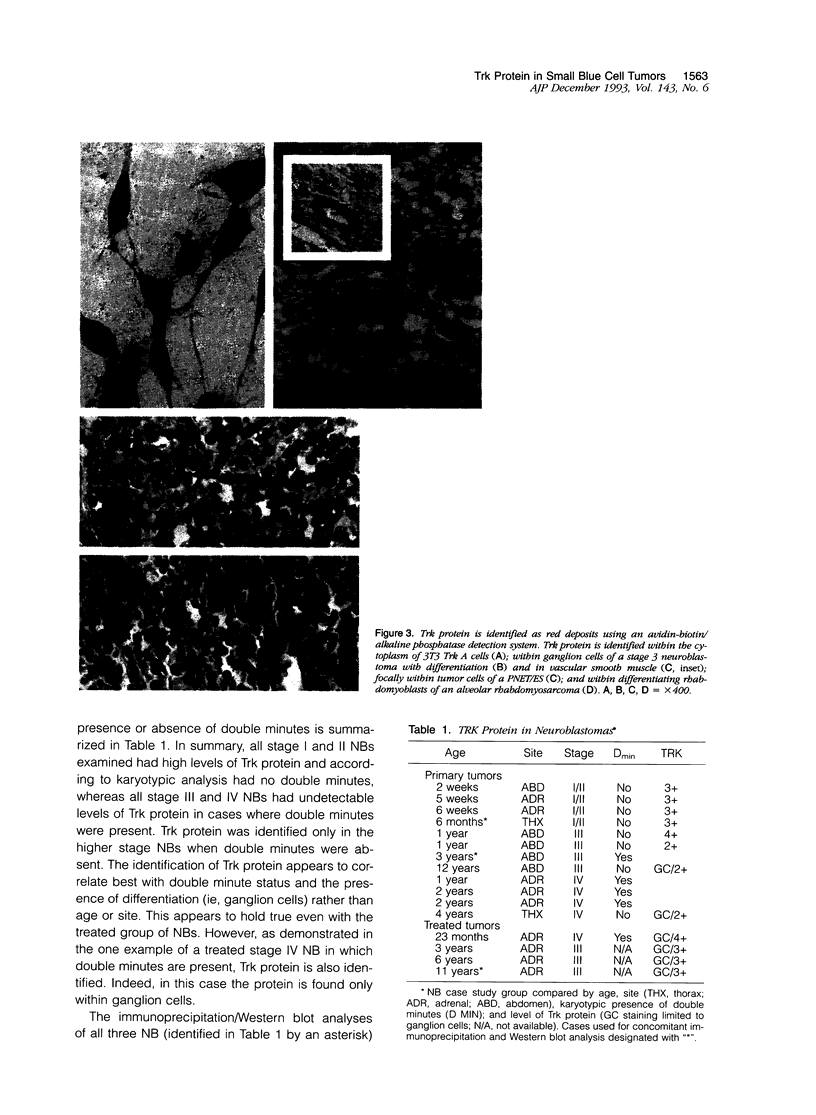
Expression of Trk protein has been documented by Northern analysis in neuroblastomas with good prognosis. To localize the expression of this protein at the cellular level within individual tumors, we adapted a recently characterized pan-Trk antibody for use in formalin fixed, paraffin-embedded tissue. We have examined a group of small round blue cell tumors occurring in children, including both high and low stage neuroblastomas, to assess the presence or absence of Trk expression and its cellular localization. Positive staining for Trk protein was observed in four of four low stage (good prognosis) neuroblastomas, five of five primitive neuroectodermal tumors/Ewing's sarcoma, five of five rhabdomyosarcomas, and no lymphomas. Within the neuroblastomas, expression of Trk protein was most striking in ganglion cells, in which positive cytoplasmic staining was demonstrated regardless of tumor stage. The latter observation may lend further insight into the pathobiology of this malignant childhood tumor.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker D. L., Reddy U. R., Pleasure S., Hardy M., Williams M., Tartaglione M., Biegel J. A., Emanuel B. S., Lo Presti P., Kreider B. Human central nervous system primitive neuroectodermal tumor expressing nerve growth factor receptors: CHP707m. Ann Neurol. 1990 Aug;28(2):136–145. doi: 10.1002/ana.410280205. [DOI] [PubMed] [Google Scholar]
- Bolande R. P. Benignity of neonatal tumors and concept of cancer repression in early life. Am J Dis Child. 1971 Jul;122(1):12–14. doi: 10.1001/archpedi.1971.02110010048004. [DOI] [PubMed] [Google Scholar]
- Brodeur G. M., Goldstein M. N. Histochemical demonstration of an increase in acetylcholinesterase in established lines of human and mouse neuroblastomas by nerve growth factor. Cytobios. 1976;16(62):133–138. [PubMed] [Google Scholar]
- Chao M. V. Neurotrophin receptors: a window into neuronal differentiation. Neuron. 1992 Oct;9(4):583–593. doi: 10.1016/0896-6273(92)90023-7. [DOI] [PubMed] [Google Scholar]
- Ernfors P., Ibáez C. F., Ebendal T., Olson L., Persson H. Molecular cloning and neurotrophic activities of a protein with structural similarities to nerve growth factor: developmental and topographical expression in the brain. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5454–5458. doi: 10.1073/pnas.87.14.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans A. E., D'Angio G. J., Randolph J. A proposed staging for children with neuroblastoma. Children's cancer study group A. Cancer. 1971 Feb;27(2):374–378. doi: 10.1002/1097-0142(197102)27:2<374::aid-cncr2820270221>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Everson T. C. Spontaneous regression of cancer. Ann N Y Acad Sci. 1964 Apr 2;114(2):721–735. [PubMed] [Google Scholar]
- Goedert M., Otten U., Hunt S. P., Bond A., Chapman D., Schlumpf M., Lichtensteiger W. Biochemical and anatomical effects of antibodies against nerve growth factor on developing rat sensory ganglia. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1580–1584. doi: 10.1073/pnas.81.5.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L. A. A quantitative bioassay for nerve growth factor (NGF) activity employing a clonal pheochromocytoma cell line. Brain Res. 1977 Sep 16;133(2):350–353. doi: 10.1016/0006-8993(77)90770-3. [DOI] [PubMed] [Google Scholar]
- Green S. H., Rydel R. E., Connolly J. L., Greene L. A. PC12 cell mutants that possess low- but not high-affinity nerve growth factor receptors neither respond to nor internalize nerve growth factor. J Cell Biol. 1986 Mar;102(3):830–843. doi: 10.1083/jcb.102.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallbök F., Ibáez C. F., Persson H. Evolutionary studies of the nerve growth factor family reveal a novel member abundantly expressed in Xenopus ovary. Neuron. 1991 May;6(5):845–858. doi: 10.1016/0896-6273(91)90180-8. [DOI] [PubMed] [Google Scholar]
- Hempstead B. L., Martin-Zanca D., Kaplan D. R., Parada L. F., Chao M. V. High-affinity NGF binding requires coexpression of the trk proto-oncogene and the low-affinity NGF receptor. Nature. 1991 Apr 25;350(6320):678–683. doi: 10.1038/350678a0. [DOI] [PubMed] [Google Scholar]
- Hempstead B. L., Martin-Zanca D., Kaplan D. R., Parada L. F., Chao M. V. High-affinity NGF binding requires coexpression of the trk proto-oncogene and the low-affinity NGF receptor. Nature. 1991 Apr 25;350(6320):678–683. doi: 10.1038/350678a0. [DOI] [PubMed] [Google Scholar]
- Hempstead B. L., Rabin S. J., Kaplan L., Reid S., Parada L. F., Kaplan D. R. Overexpression of the trk tyrosine kinase rapidly accelerates nerve growth factor-induced differentiation. Neuron. 1992 Nov;9(5):883–896. doi: 10.1016/0896-6273(92)90241-5. [DOI] [PubMed] [Google Scholar]
- Hempstead B. L., Schleifer L. S., Chao M. V. Expression of functional nerve growth factor receptors after gene transfer. Science. 1989 Jan 20;243(4889):373–375. doi: 10.1126/science.2536190. [DOI] [PubMed] [Google Scholar]
- Holtzman D. M., Li Y., Parada L. F., Kinsman S., Chen C. K., Valletta J. S., Zhou J., Long J. B., Mobley W. C. p140trk mRNA marks NGF-responsive forebrain neurons: evidence that trk gene expression is induced by NGF. Neuron. 1992 Sep;9(3):465–478. doi: 10.1016/0896-6273(92)90184-f. [DOI] [PubMed] [Google Scholar]
- Jensen L. M. Phenotypic differentiation of aphidicolin-selected human neuroblastoma cultures after long-term exposure to nerve growth factor. Dev Biol. 1987 Mar;120(1):56–64. doi: 10.1016/0012-1606(87)90103-5. [DOI] [PubMed] [Google Scholar]
- Kaplan D. R., Hempstead B. L., Martin-Zanca D., Chao M. V., Parada L. F. The trk proto-oncogene product: a signal transducing receptor for nerve growth factor. Science. 1991 Apr 26;252(5005):554–558. doi: 10.1126/science.1850549. [DOI] [PubMed] [Google Scholar]
- Klein R., Conway D., Parada L. F., Barbacid M. The trkB tyrosine protein kinase gene codes for a second neurogenic receptor that lacks the catalytic kinase domain. Cell. 1990 May 18;61(4):647–656. doi: 10.1016/0092-8674(90)90476-u. [DOI] [PubMed] [Google Scholar]
- Klein R., Jing S. Q., Nanduri V., O'Rourke E., Barbacid M. The trk proto-oncogene encodes a receptor for nerve growth factor. Cell. 1991 Apr 5;65(1):189–197. doi: 10.1016/0092-8674(91)90419-y. [DOI] [PubMed] [Google Scholar]
- Klein R., Lamballe F., Bryant S., Barbacid M. The trkB tyrosine protein kinase is a receptor for neurotrophin-4. Neuron. 1992 May;8(5):947–956. doi: 10.1016/0896-6273(92)90209-v. [DOI] [PubMed] [Google Scholar]
- Lamballe F., Klein R., Barbacid M. trkC, a new member of the trk family of tyrosine protein kinases, is a receptor for neurotrophin-3. Cell. 1991 Sep 6;66(5):967–979. doi: 10.1016/0092-8674(91)90442-2. [DOI] [PubMed] [Google Scholar]
- Landreth G. E., Shooter E. M. Nerve growth factor receptors on PC12 cells: ligand-induced conversion from low- to high-affinity states. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4751–4755. doi: 10.1073/pnas.77.8.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibrock J., Lottspeich F., Hohn A., Hofer M., Hengerer B., Masiakowski P., Thoenen H., Barde Y. A. Molecular cloning and expression of brain-derived neurotrophic factor. Nature. 1989 Sep 14;341(6238):149–152. doi: 10.1038/341149a0. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor: thirty-five years later. EMBO J. 1987 May;6(5):1145–1154. doi: 10.1002/j.1460-2075.1987.tb02347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb D. M., Maragos J., Martin-Zanca D., Chao M. V., Parada L. F., Greene L. A. The trk proto-oncogene rescues NGF responsiveness in mutant NGF-nonresponsive PC12 cell lines. Cell. 1991 Sep 6;66(5):961–966. doi: 10.1016/0092-8674(91)90441-z. [DOI] [PubMed] [Google Scholar]
- Martin-Zanca D., Barbacid M., Parada L. F. Expression of the trk proto-oncogene is restricted to the sensory cranial and spinal ganglia of neural crest origin in mouse development. Genes Dev. 1990 May;4(5):683–694. doi: 10.1101/gad.4.5.683. [DOI] [PubMed] [Google Scholar]
- Martin-Zanca D., Oskam R., Mitra G., Copeland T., Barbacid M. Molecular and biochemical characterization of the human trk proto-oncogene. Mol Cell Biol. 1989 Jan;9(1):24–33. doi: 10.1128/mcb.9.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima H., Bogenmann E. Nerve growth factor (NGF) induces neuronal differentiation in neuroblastoma cells transfected with the NGF receptor cDNA. Mol Cell Biol. 1990 Sep;10(9):5015–5020. doi: 10.1128/mcb.10.9.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawara A., Arima-Nakagawara M., Scavarda N. J., Azar C. G., Cantor A. B., Brodeur G. M. Association between high levels of expression of the TRK gene and favorable outcome in human neuroblastoma. N Engl J Med. 1993 Mar 25;328(12):847–854. doi: 10.1056/NEJM199303253281205. [DOI] [PubMed] [Google Scholar]
- Nakagawara A., Arima M., Azar C. G., Scavarda N. J., Brodeur G. M. Inverse relationship between trk expression and N-myc amplification in human neuroblastomas. Cancer Res. 1992 Mar 1;52(5):1364–1368. [PubMed] [Google Scholar]
- Scarisbrick I. A., Jones E. G., Isackson P. J. Coexpression of mRNAs for NGF, BDNF, and NT-3 in the cardiovascular system of the pre- and postnatal rat. J Neurosci. 1993 Mar;13(3):875–893. doi: 10.1523/JNEUROSCI.13-03-00875.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter A. L., Bothwell M. A. Nerve growth factor receptors on PC12 cells: evidence for two receptor classes with differing cytoskeletal association. Cell. 1981 Jun;24(3):867–874. doi: 10.1016/0092-8674(81)90112-4. [DOI] [PubMed] [Google Scholar]
- Snider W. D., Elliott J. L., Yan Q. Axotomy-induced neuronal death during development. J Neurobiol. 1992 Nov;23(9):1231–1246. doi: 10.1002/neu.480230913. [DOI] [PubMed] [Google Scholar]
- Sonnenfeld K. H., Ishii D. N. Fast and slow nerve growth factor binding sites in human neuroblastoma and rat pheochromocytoma cell lines: relationship of sites to each other and to neurite formation. J Neurosci. 1985 Jul;5(7):1717–1728. doi: 10.1523/JNEUROSCI.05-07-01717.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter A., Riopelle R. J., Harris-Warrick R. M., Shooter E. M. Nerve growth factor receptors. Characterization of two distinct classes of binding sites on chick embryo sensory ganglia cells. J Biol Chem. 1979 Jul 10;254(13):5972–5982. [PubMed] [Google Scholar]
- Thoenen H., Barde Y. A. Physiology of nerve growth factor. Physiol Rev. 1980 Oct;60(4):1284–1335. doi: 10.1152/physrev.1980.60.4.1284. [DOI] [PubMed] [Google Scholar]
- Thomson T. M., Pellicer A., Greene L. A. Functional receptors for nerve growth factor on Ewing's sarcoma and Wilm's tumor cells. J Cell Physiol. 1989 Oct;141(1):60–64. doi: 10.1002/jcp.1041410110. [DOI] [PubMed] [Google Scholar]
- Tsoulfas P., Soppet D., Escandon E., Tessarollo L., Mendoza-Ramirez J. L., Rosenthal A., Nikolics K., Parada L. F. The rat trkC locus encodes multiple neurogenic receptors that exhibit differential response to neurotrophin-3 in PC12 cells. Neuron. 1993 May;10(5):975–990. doi: 10.1016/0896-6273(93)90212-a. [DOI] [PubMed] [Google Scholar]
- Valenzuela D. M., Maisonpierre P. C., Glass D. J., Rojas E., Nuñez L., Kong Y., Gies D. R., Stitt T. N., Ip N. Y., Yancopoulos G. D. Alternative forms of rat TrkC with different functional capabilities. Neuron. 1993 May;10(5):963–974. doi: 10.1016/0896-6273(93)90211-9. [DOI] [PubMed] [Google Scholar]