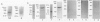Abstract
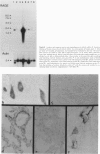
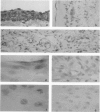
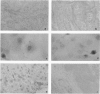
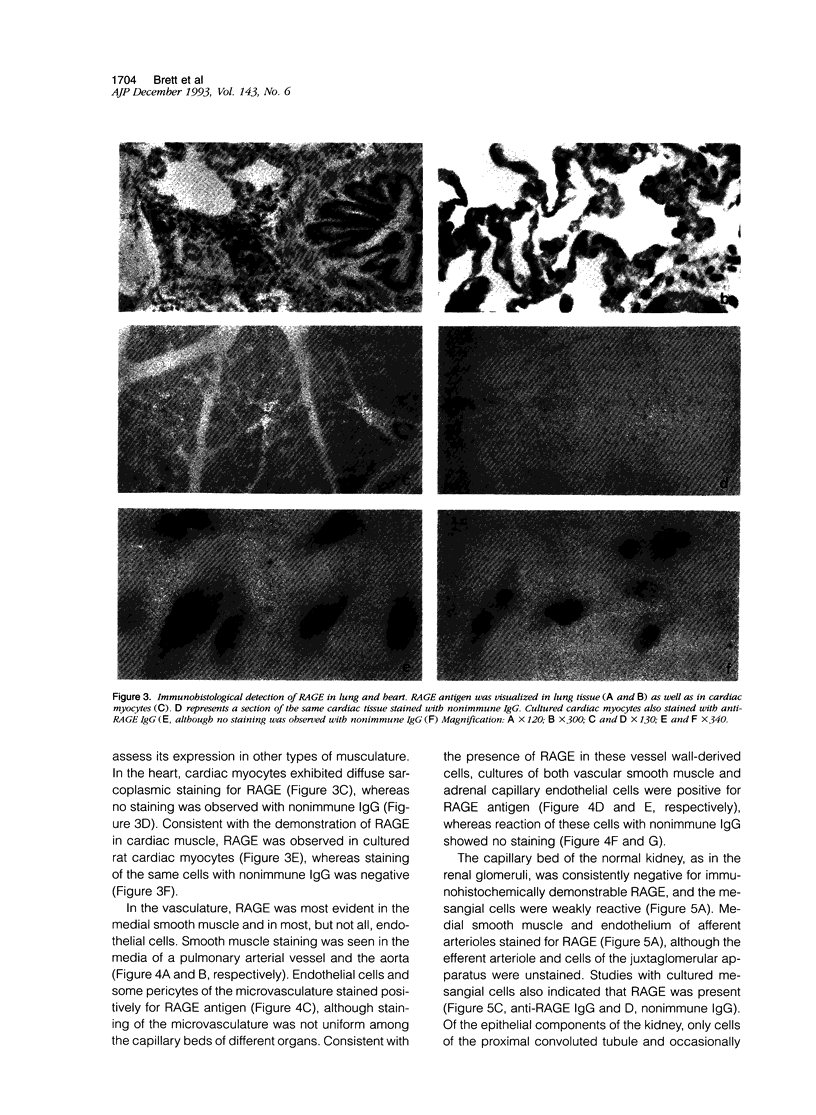
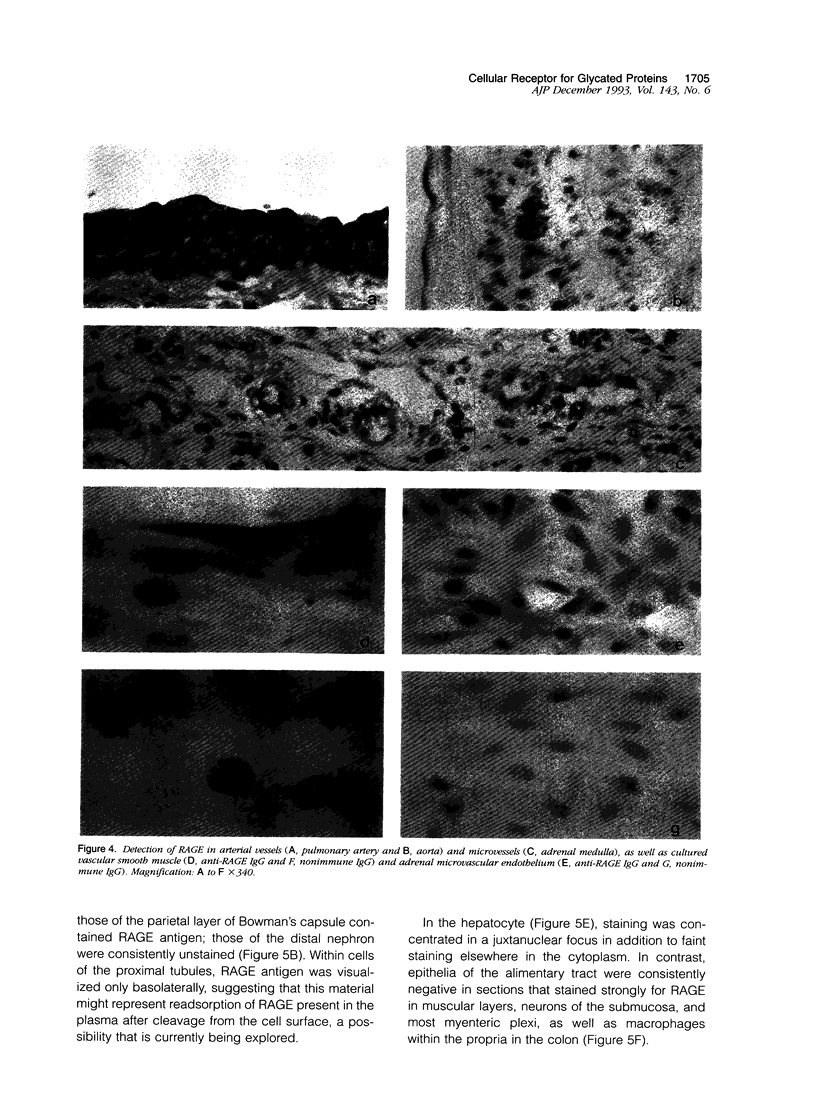
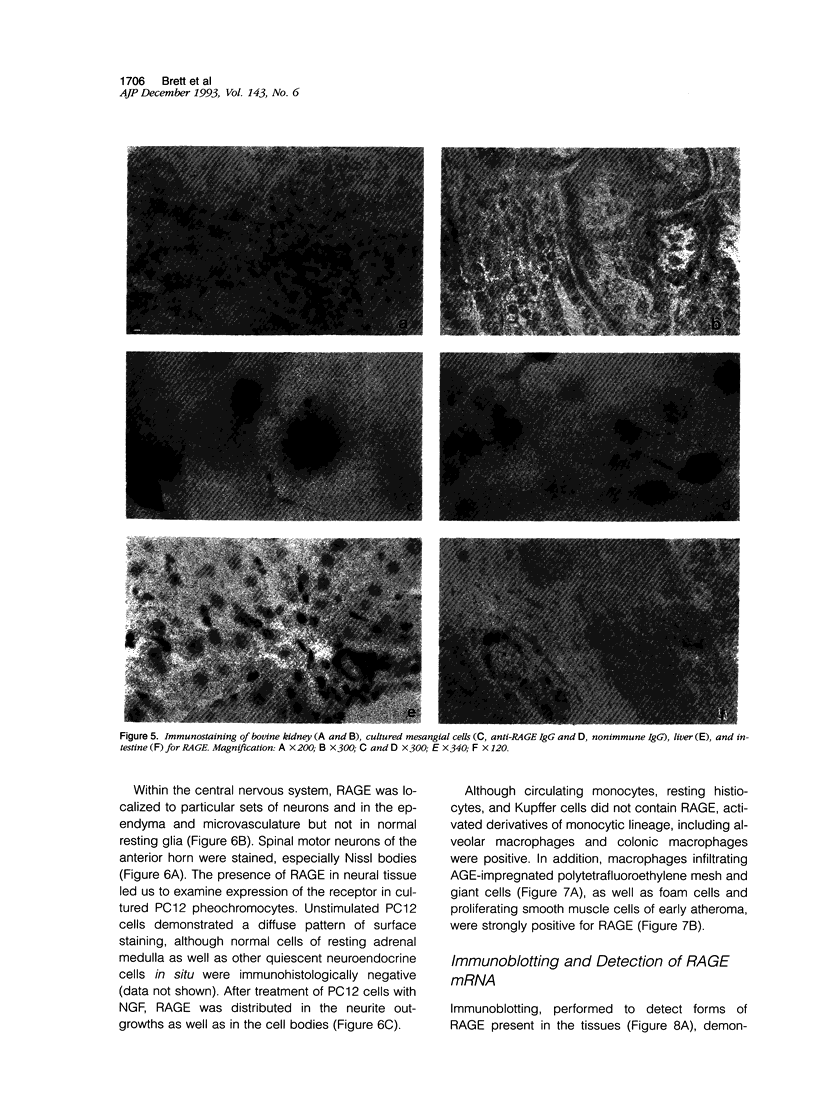
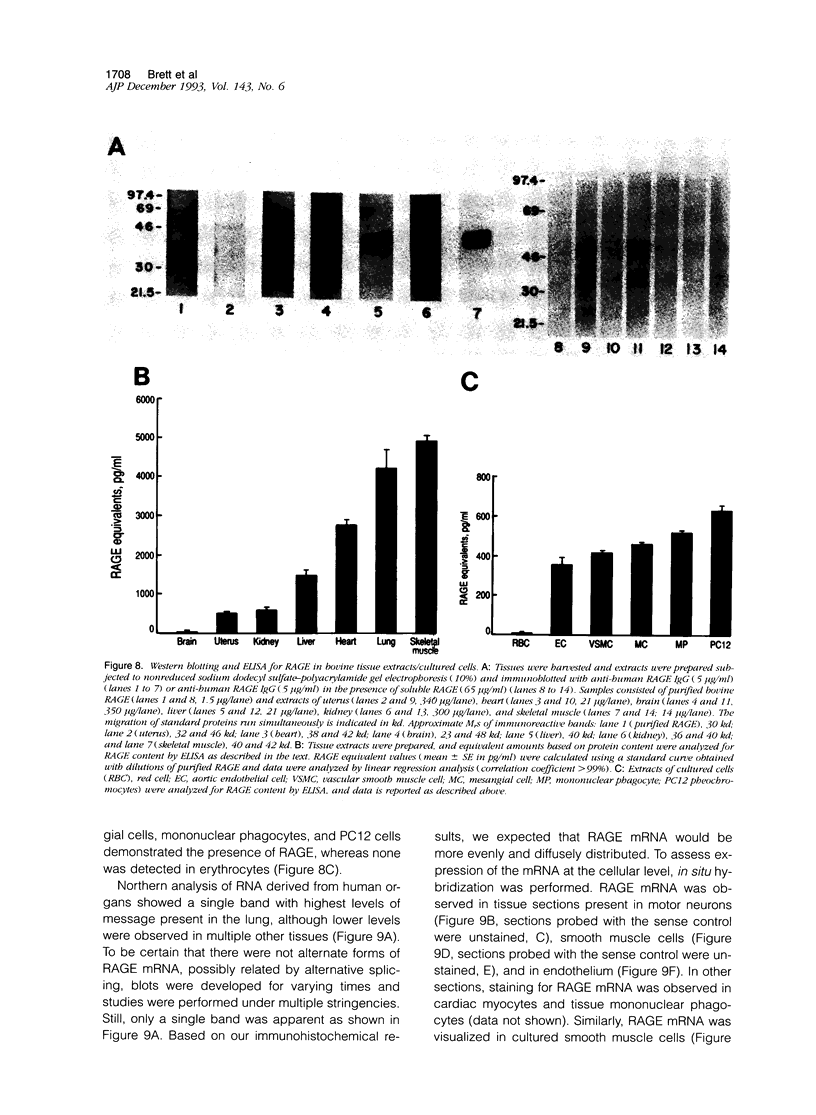
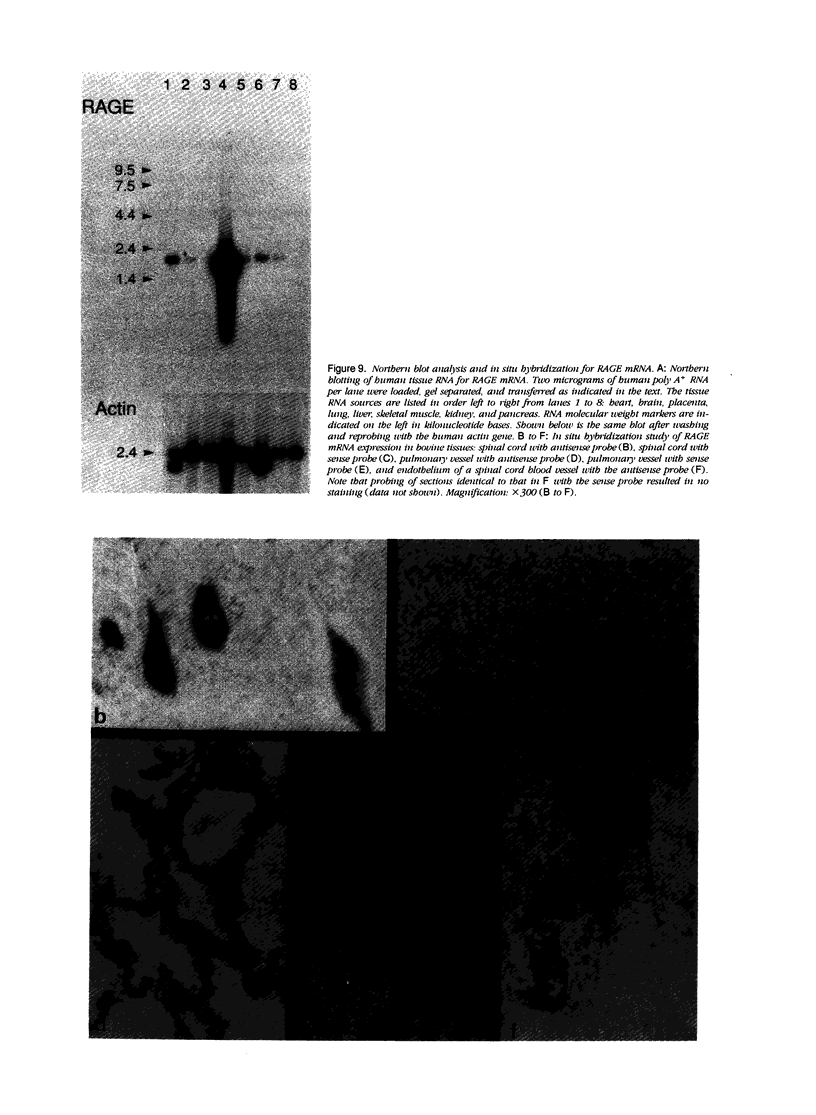
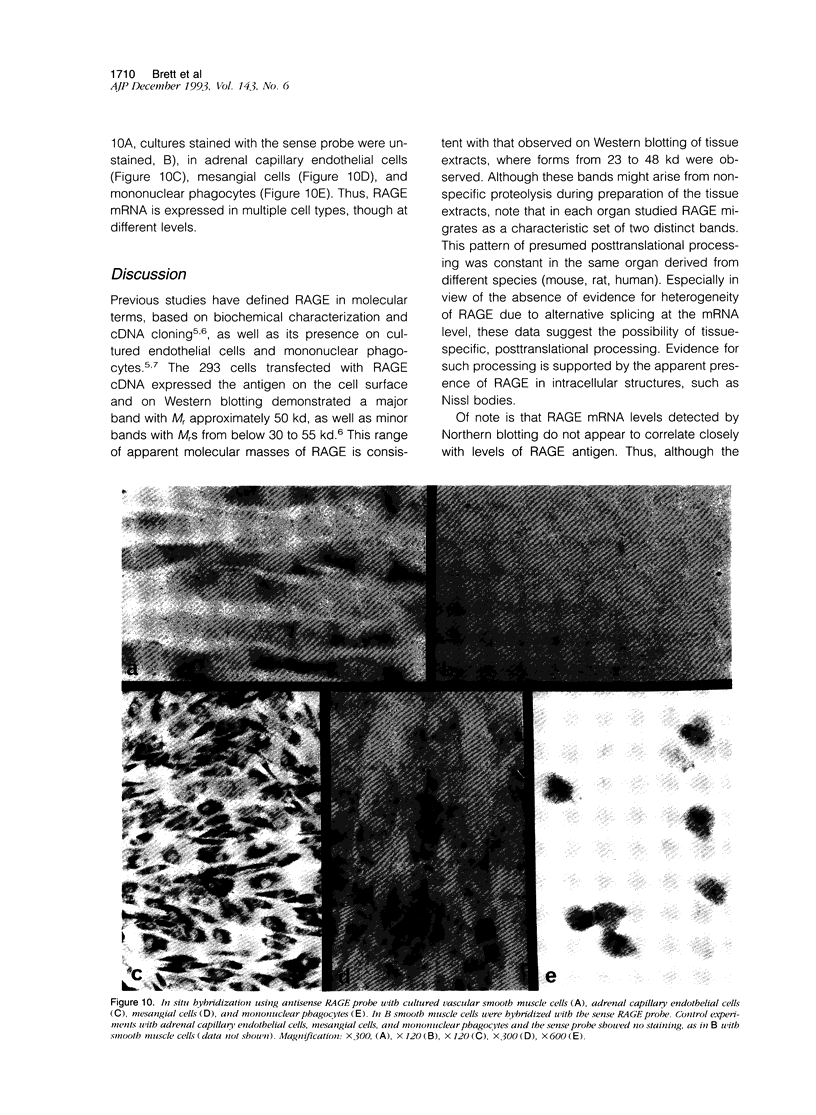
Advanced glycation end products (AGEs), the final products of nonenzymatic glycation and oxidation of proteins, are found in the plasma and accumulate in the tissues during aging and at an accelerated rate in diabetes. A novel integral membrane protein, termed receptor for AGE (RAGE), forms a central part of the cell surface binding site for AGEs. Using monospecific, polyclonal antibody raised to human recombinant and bovine RAGE, immunostaining of bovine tissues showed RAGE in the vasculature, endothelium, and smooth muscle cells and in mononuclear cells in the tissues. Consistent with these data, RAGE antigen and mRNA were identified in cultured bovine endothelium, vascular smooth muscle, and monocyte-derived macrophages. RAGE antigen was also visualized in bovine cardiac myocytes as well as in cultures of neonatal rat cardiac myocytes and in neural tissue where motor neurons, peripheral nerves, and a population of cortical neurons were positive. In situ hybridization confirmed the presence of RAGE mRNA in the tissues, and studies with rat PC12 pheochromocytes indicated that they provide a neuronal-related cell culture model for examining RAGE expression. Pathological studies of human atherosclerotic plaques showed infiltration of RAGE-expressing cells in the expanded intima. These results indicate that RAGE is present in multiple tissues and suggest the potential relevance of AGE-RAGE interactions for modulating properties of the vasculature as well as neural and cardiac function, prominent areas of involvement in diabetes and in the normal aging process.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brownlee M., Cerami A., Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988 May 19;318(20):1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- Dyer D. G., Blackledge J. A., Thorpe S. R., Baynes J. W. Formation of pentosidine during nonenzymatic browning of proteins by glucose. Identification of glucose and other carbohydrates as possible precursors of pentosidine in vivo. J Biol Chem. 1991 Jun 25;266(18):11654–11660. [PubMed] [Google Scholar]
- Esposito C., Gerlach H., Brett J., Stern D., Vlassara H. Endothelial receptor-mediated binding of glucose-modified albumin is associated with increased monolayer permeability and modulation of cell surface coagulant properties. J Exp Med. 1989 Oct 1;170(4):1387–1407. doi: 10.1084/jem.170.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furie M. B., Cramer E. B., Naprstek B. L., Silverstein S. C. Cultured endothelial cell monolayers that restrict the transendothelial passage of macromolecules and electrical current. J Cell Biol. 1984 Mar;98(3):1033–1041. doi: 10.1083/jcb.98.3.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M., Gordon D., Lu P. L. A smooth muscle-specific monoclonal antibody recognizes smooth muscle actin isozymes. J Cell Biol. 1985 Mar;100(3):807–813. doi: 10.1083/jcb.100.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene L. A., Aletta J. M., Rukenstein A., Green S. H. PC12 pheochromocytoma cells: culture, nerve growth factor treatment, and experimental exploitation. Methods Enzymol. 1987;147:207–216. doi: 10.1016/0076-6879(87)47111-5. [DOI] [PubMed] [Google Scholar]
- Lau Y. H., Robinson R. B., Rosen M. R., Bilezikian J. P. Subclassification of beta-adrenergic receptors in cultured rat cardiac myoblasts and fibroblasts. Circ Res. 1980 Jul;47(1):41–48. doi: 10.1161/01.res.47.1.41. [DOI] [PubMed] [Google Scholar]
- Makita Z., Vlassara H., Cerami A., Bucala R. Immunochemical detection of advanced glycosylation end products in vivo. J Biol Chem. 1992 Mar 15;267(8):5133–5138. [PubMed] [Google Scholar]
- Neeper M., Schmidt A. M., Brett J., Yan S. D., Wang F., Pan Y. C., Elliston K., Stern D., Shaw A. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem. 1992 Jul 25;267(21):14998–15004. [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Schindler R., Gelfand J. A., Dinarello C. A. Recombinant C5a stimulates transcription rather than translation of interleukin-1 (IL-1) and tumor necrosis factor: translational signal provided by lipopolysaccharide or IL-1 itself. Blood. 1990 Oct 15;76(8):1631–1638. [PubMed] [Google Scholar]
- Schmidt A. M., Vianna M., Gerlach M., Brett J., Ryan J., Kao J., Esposito C., Hegarty H., Hurley W., Clauss M. Isolation and characterization of two binding proteins for advanced glycosylation end products from bovine lung which are present on the endothelial cell surface. J Biol Chem. 1992 Jul 25;267(21):14987–14997. [PubMed] [Google Scholar]
- Schmidt A. M., Yan S. D., Brett J., Mora R., Nowygrod R., Stern D. Regulation of human mononuclear phagocyte migration by cell surface-binding proteins for advanced glycation end products. J Clin Invest. 1993 May;91(5):2155–2168. doi: 10.1172/JCI116442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell D. R., Monnier V. M. Structure elucidation of a senescence cross-link from human extracellular matrix. Implication of pentoses in the aging process. J Biol Chem. 1989 Dec 25;264(36):21597–21602. [PubMed] [Google Scholar]
- Skolnik E. Y., Yang Z., Makita Z., Radoff S., Kirstein M., Vlassara H. Human and rat mesangial cell receptors for glucose-modified proteins: potential role in kidney tissue remodelling and diabetic nephropathy. J Exp Med. 1991 Oct 1;174(4):931–939. doi: 10.1084/jem.174.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaitukaitis J. L. Production of antisera with small doses of immunogen: multiple intradermal injections. Methods Enzymol. 1981;73(Pt B):46–52. doi: 10.1016/0076-6879(81)73055-6. [DOI] [PubMed] [Google Scholar]
- Yang Z., Makita Z., Horii Y., Brunelle S., Cerami A., Sehajpal P., Suthanthiran M., Vlassara H. Two novel rat liver membrane proteins that bind advanced glycosylation endproducts: relationship to macrophage receptor for glucose-modified proteins. J Exp Med. 1991 Sep 1;174(3):515–524. doi: 10.1084/jem.174.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]