Abstract
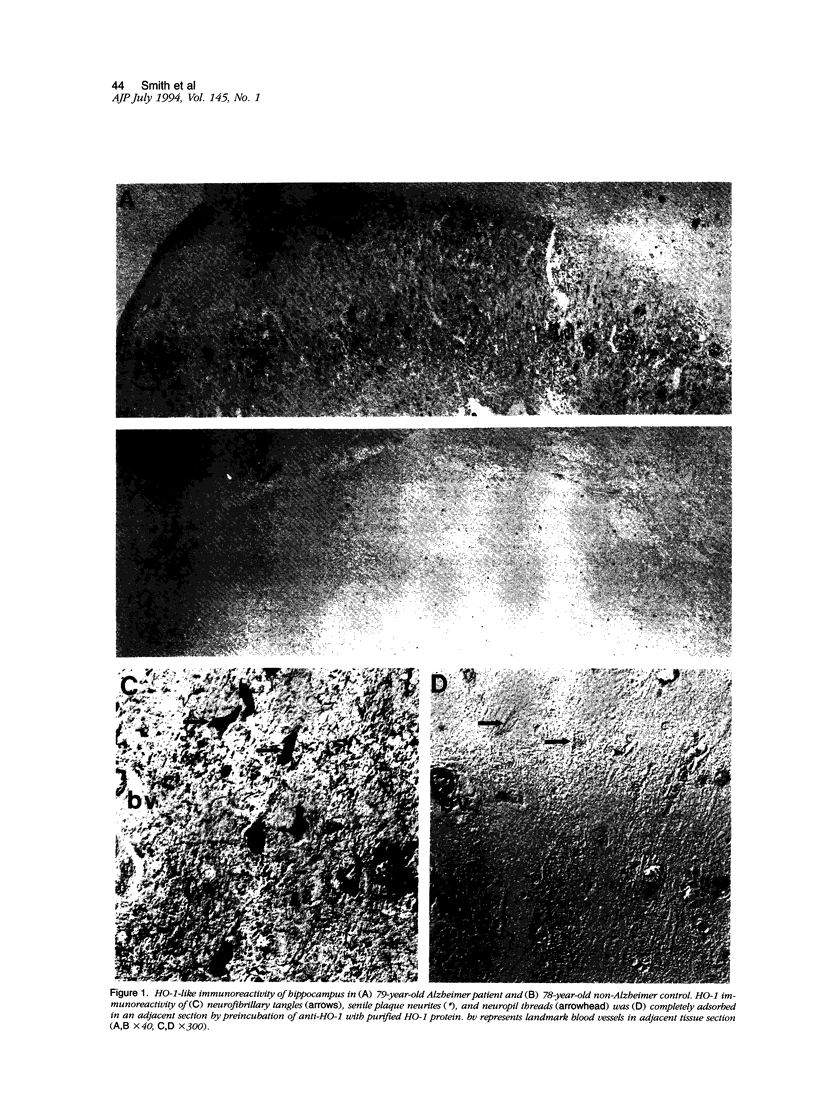
Heme oxygenase-1 is an important enzyme that degrades heme, a pro-oxidant, leading to the formation of antioxidant molecules. In this study we demonstrate by immunocytochemistry close association of heme oxygenase-1 with Alzheimer neurofibrillary pathology and with the neurofibrillary tangles found in progressive supranuclear palsy and subacute sclerosing panencephalitis. In Alzheimer's disease, using two different rabbit antisera against heme oxygenase-1 protein, we localized, using immunocytochemical methods, heme oxygenase-1 to neurofibrillary tangles, senile plaque neurites, granulovacuolar degeneration, and neuropil threads. Only light background staining was seen in young controls and sporadic lesion-related immunoreactivity in age-matched controls. The increase in heme oxygenase-1 protein in association with the neurofibrillary pathology of Alzheimer's disease and other diseases characterized by neurofibrillary tangles supports the notion that the generation of free radicals and oxidative stress plays a role in the pathogenesis of neurofibrillary pathology.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. D., Jr, Klaidman L. K., Odunze I. N., Shen H. C., Miller C. A. Alzheimer's and Parkinson's disease. Brain levels of glutathione, glutathione disulfide, and vitamin E. Mol Chem Neuropathol. 1991 Jun;14(3):213–226. doi: 10.1007/BF03159937. [DOI] [PubMed] [Google Scholar]
- Corder E. H., Saunders A. M., Strittmatter W. J., Schmechel D. E., Gaskell P. C., Small G. W., Roses A. D., Haines J. L., Pericak-Vance M. A. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993 Aug 13;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Cross C. E., Halliwell B., Borish E. T., Pryor W. A., Ames B. N., Saul R. L., McCord J. M., Harman D. Oxygen radicals and human disease. Ann Intern Med. 1987 Oct;107(4):526–545. doi: 10.7326/0003-4819-107-4-526. [DOI] [PubMed] [Google Scholar]
- Cruse I., Maines M. D. Evidence suggesting that the two forms of heme oxygenase are products of different genes. J Biol Chem. 1988 Mar 5;263(7):3348–3353. [PubMed] [Google Scholar]
- Ewing J. F., Haber S. N., Maines M. D. Normal and heat-induced patterns of expression of heme oxygenase-1 (HSP32) in rat brain: hyperthermia causes rapid induction of mRNA and protein. J Neurochem. 1992 Mar;58(3):1140–1149. doi: 10.1111/j.1471-4159.1992.tb09373.x. [DOI] [PubMed] [Google Scholar]
- Ewing J. F., Maines M. D. Glutathione depletion induces heme oxygenase-1 (HSP32) mRNA and protein in rat brain. J Neurochem. 1993 Apr;60(4):1512–1519. doi: 10.1111/j.1471-4159.1993.tb03315.x. [DOI] [PubMed] [Google Scholar]
- Gupta S., Rifici V., Crowley S., Brownlee M., Shan Z., Schlondorff D. Interactions of LDL and modified LDL with mesangial cells and matrix. Kidney Int. 1992 May;41(5):1161–1169. doi: 10.1038/ki.1992.177. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- Hamos J. E., Oblas B., Pulaski-Salo D., Welch W. J., Bole D. G., Drachman D. A. Expression of heat shock proteins in Alzheimer's disease. Neurology. 1991 Mar;41(3):345–350. doi: 10.1212/wnl.41.3.345. [DOI] [PubMed] [Google Scholar]
- Hunt J. V., Wolff S. P. Oxidative glycation and free radical production: a causal mechanism of diabetic complications. Free Radic Res Commun. 1991;12-13 Pt 1:115–123. doi: 10.3109/10715769109145775. [DOI] [PubMed] [Google Scholar]
- Keyse S. M., Applegate L. A., Tromvoukis Y., Tyrrell R. M. Oxidant stress leads to transcriptional activation of the human heme oxygenase gene in cultured skin fibroblasts. Mol Cell Biol. 1990 Sep;10(9):4967–4969. doi: 10.1128/mcb.10.9.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyse S. M., Tyrrell R. M. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl Acad Sci U S A. 1989 Jan;86(1):99–103. doi: 10.1073/pnas.86.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyse S. M., Tyrrell R. M. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl Acad Sci U S A. 1989 Jan;86(1):99–103. doi: 10.1073/pnas.86.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish S. J., Morito C. L., Hornykiewicz O. Brain glutathione peroxidase in neurodegenerative disorders. Neurochem Pathol. 1986 Feb;4(1):23–28. doi: 10.1007/BF02834296. [DOI] [PubMed] [Google Scholar]
- Levere R. D., Staudinger R., Loewy G., Kappas A., Shibahara S., Abraham N. G. Elevated levels of heme oxygenase-1 activity and mRNA in peripheral blood adherent cells of acquired immunodeficiency syndrome patients. Am J Hematol. 1993 May;43(1):19–23. doi: 10.1002/ajh.2830430106. [DOI] [PubMed] [Google Scholar]
- Lowe J., McDermott H., Pike I., Spendlove I., Landon M., Mayer R. J. alpha B crystallin expression in non-lenticular tissues and selective presence in ubiquitinated inclusion bodies in human disease. J Pathol. 1992 Jan;166(1):61–68. doi: 10.1002/path.1711660110. [DOI] [PubMed] [Google Scholar]
- Maines M. D. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988 Jul;2(10):2557–2568. [PubMed] [Google Scholar]
- Maines M. D., Trakshel G. M., Kutty R. K. Characterization of two constitutive forms of rat liver microsomal heme oxygenase. Only one molecular species of the enzyme is inducible. J Biol Chem. 1986 Jan 5;261(1):411–419. [PubMed] [Google Scholar]
- Mori H., Kondo J., Ihara Y. Ubiquitin is a component of paired helical filaments in Alzheimer's disease. Science. 1987 Mar 27;235(4796):1641–1644. doi: 10.1126/science.3029875. [DOI] [PubMed] [Google Scholar]
- Pearson R. C., Esiri M. M., Hiorns R. W., Wilcock G. K., Powell T. P. Anatomical correlates of the distribution of the pathological changes in the neocortex in Alzheimer disease. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4531–4534. doi: 10.1073/pnas.82.13.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry E. K., Atack J. R., Perry R. H., Hardy J. A., Dodd P. R., Edwardson J. A., Blessed G., Tomlinson B. E., Fairbairn A. F. Intralaminar neurochemical distributions in human midtemporal cortex: comparison between Alzheimer's disease and the normal. J Neurochem. 1984 May;42(5):1402–1410. doi: 10.1111/j.1471-4159.1984.tb02801.x. [DOI] [PubMed] [Google Scholar]
- Perry G., Friedman R., Shaw G., Chau V. Ubiquitin is detected in neurofibrillary tangles and senile plaque neurites of Alzheimer disease brains. Proc Natl Acad Sci U S A. 1987 May;84(9):3033–3036. doi: 10.1073/pnas.84.9.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry G., Kawai M., Tabaton M., Onorato M., Mulvihill P., Richey P., Morandi A., Connolly J. A., Gambetti P. Neuropil threads of Alzheimer's disease show a marked alteration of the normal cytoskeleton. J Neurosci. 1991 Jun;11(6):1748–1755. doi: 10.1523/JNEUROSCI.11-06-01748.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry T. L., Yong V. W., Bergeron C., Hansen S., Jones K. Amino acids, glutathione, and glutathione transferase activity in the brains of patients with Alzheimer's disease. Ann Neurol. 1987 Apr;21(4):331–336. doi: 10.1002/ana.410210403. [DOI] [PubMed] [Google Scholar]
- Posnett D. N., McGrath H., Tam J. P. A novel method for producing anti-peptide antibodies. Production of site-specific antibodies to the T cell antigen receptor beta-chain. J Biol Chem. 1988 Feb 5;263(4):1719–1725. [PubMed] [Google Scholar]
- Renkawek K., Bosman G. J., Gaestel M. Increased expression of heat-shock protein 27 kDa in Alzheimer disease: a preliminary study. Neuroreport. 1993 Oct 25;5(1):14–16. doi: 10.1097/00001756-199310000-00003. [DOI] [PubMed] [Google Scholar]
- Rotenberg M. O., Maines M. D. Isolation, characterization, and expression in Escherichia coli of a cDNA encoding rat heme oxygenase-2. J Biol Chem. 1990 May 5;265(13):7501–7506. [PubMed] [Google Scholar]
- Selkoe D. J., Ihara Y., Salazar F. J. Alzheimer's disease: insolubility of partially purified paired helical filaments in sodium dodecyl sulfate and urea. Science. 1982 Mar 5;215(4537):1243–1245. doi: 10.1126/science.6120571. [DOI] [PubMed] [Google Scholar]
- Shibahara S., Müller R. M., Taguchi H. Transcriptional control of rat heme oxygenase by heat shock. J Biol Chem. 1987 Sep 25;262(27):12889–12892. [PubMed] [Google Scholar]
- Shibahara S., Müller R., Taguchi H., Yoshida T. Cloning and expression of cDNA for rat heme oxygenase. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7865–7869. doi: 10.1073/pnas.82.23.7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. A., Taneda S., Richey P. L., Miyata S., Yan S. D., Stern D., Sayre L. M., Monnier V. M., Perry G. Advanced Maillard reaction end products are associated with Alzheimer disease pathology. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5710–5714. doi: 10.1073/pnas.91.12.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker R., Yamamoto Y., McDonagh A. F., Glazer A. N., Ames B. N. Bilirubin is an antioxidant of possible physiological importance. Science. 1987 Feb 27;235(4792):1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- Strittmatter W. J., Weisgraber K. H., Huang D. Y., Dong L. M., Salvesen G. S., Pericak-Vance M., Schmechel D., Saunders A. M., Goldgaber D., Roses A. D. Binding of human apolipoprotein E to synthetic amyloid beta peptide: isoform-specific effects and implications for late-onset Alzheimer disease. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8098–8102. doi: 10.1073/pnas.90.17.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Rotenberg M. O., Maines M. D. Developmental expression of heme oxygenase isozymes in rat brain. Two HO-2 mRNAs are detected. J Biol Chem. 1990 May 15;265(14):8212–8217. [PubMed] [Google Scholar]
- Trakshel G. M., Kutty R. K., Maines M. D. Resolution of the rat brain heme oxygenase activity: absence of a detectable amount of the inducible form (HO-1). Arch Biochem Biophys. 1988 Feb 1;260(2):732–739. doi: 10.1016/0003-9861(88)90503-6. [DOI] [PubMed] [Google Scholar]
- Trakshel G. M., Maines M. D. Multiplicity of heme oxygenase isozymes. HO-1 and HO-2 are different molecular species in rat and rabbit. J Biol Chem. 1989 Jan 15;264(2):1323–1328. [PubMed] [Google Scholar]
- Tuite M. F., Bossier P., Fitch I. T. A highly conserved sequence in yeast heat shock gene promoters. Nucleic Acids Res. 1988 Dec 23;16(24):11845–11845. doi: 10.1093/nar/16.24.11845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell R. M., Applegate L. A., Tromvoukis Y. The proximal promoter region of the human heme oxygenase gene contains elements involved in stimulation of transcriptional activity by a variety of agents including oxidants. Carcinogenesis. 1993 Apr;14(4):761–765. doi: 10.1093/carcin/14.4.761. [DOI] [PubMed] [Google Scholar]
- Verma A., Hirsch D. J., Glatt C. E., Ronnett G. V., Snyder S. H. Carbon monoxide: a putative neural messenger. Science. 1993 Jan 15;259(5093):381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]



